Sketch an energy level diagram for this overall reaction, which is exothermic. Add to your diagram a second sketch for the uncatalyzed reaction. Clearly label which curve is catalyzed, and which is uncatalyzed. Also label the activation energy (EA) and the AH for each reaction. Use your diagram to explain why one of these reactions is faster than the other, even though they start and end in the same place.
Sketch an energy level diagram for this overall reaction, which is exothermic. Add to your diagram a second sketch for the uncatalyzed reaction. Clearly label which curve is catalyzed, and which is uncatalyzed. Also label the activation energy (EA) and the AH for each reaction. Use your diagram to explain why one of these reactions is faster than the other, even though they start and end in the same place.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section: Chapter Questions
Problem 59QRT
Related questions
Question

Transcribed Image Text:Sketch an energy level diagram for this overall reaction, which is exothermic. Add to your diagram a
second sketch for the uncatalyzed reaction. Clearly label which curve is catalyzed, and which is
uncatalyzed. Also label the activation energy (EA) and the AH for each reaction. Use your diagram to
explain why one of these reactions is faster than the other, even though they start and end in the same
place.
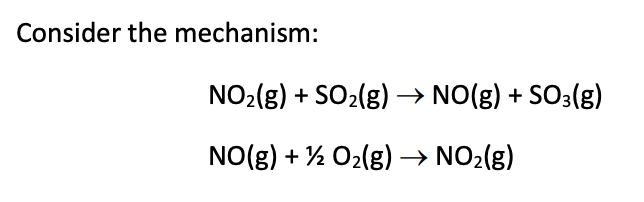
Transcribed Image Text:Consider the mechanism:
NO2(8) + SO2(g) → NO(g) + SO3(g)
NO(g) + ½ O2(g) –→ NO2(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning