A catalyst provides an alternate pathway for a reaction to occur. The alternate pathway has a lower activation energy (AE), meaning that the reactant molecules do not need to Chemical Potential collide with as much energy in order for a reaction to occur, so more collisions result in a reaction, so the Energy (Enthalpy) reaction rate is faster. Activation Energy (AE) with a catalyst Note that adding a catalyst does not change the amount of energy released (AH) for the reaction. PE of Reactants AH.. PE of Products Rxn Progress What effect does a catalyst have on the stoichiometry of the reaction? What effect does the catalyst have on the mechanism of a reaction? AE wlo a catalyfst
A catalyst provides an alternate pathway for a reaction to occur. The alternate pathway has a lower activation energy (AE), meaning that the reactant molecules do not need to Chemical Potential collide with as much energy in order for a reaction to occur, so more collisions result in a reaction, so the Energy (Enthalpy) reaction rate is faster. Activation Energy (AE) with a catalyst Note that adding a catalyst does not change the amount of energy released (AH) for the reaction. PE of Reactants AH.. PE of Products Rxn Progress What effect does a catalyst have on the stoichiometry of the reaction? What effect does the catalyst have on the mechanism of a reaction? AE wlo a catalyfst
Chapter12: Chemical Kinetics
Section: Chapter Questions
Problem 9RQ
Related questions
Question
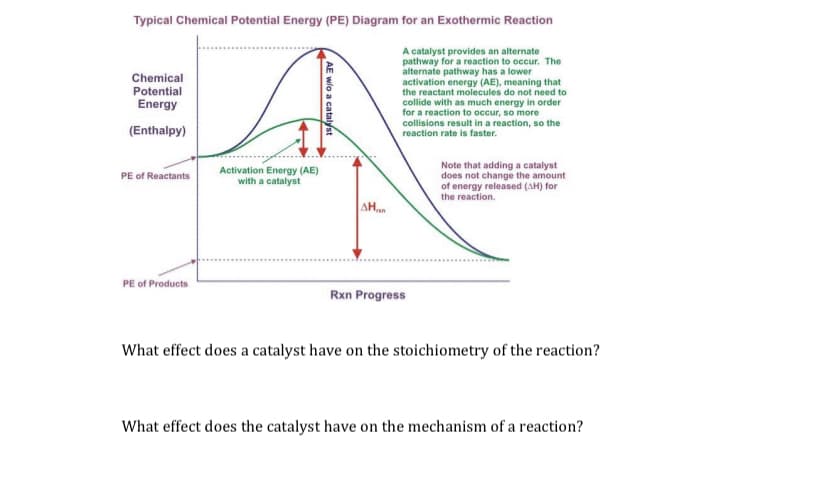
Transcribed Image Text:Typical Chemical Potential Energy (PE) Diagram for an Exothermic Reaction
A catalyst provides an alternate
pathway for a reaction to occur. The
alternate pathway has a lower
activation energy (AE), meaning that
the reactant molecules do not need to
collide with as much energy in order
for a reaction to occur, so more
collisions result in a reaction, so the
reaction rate is faster.
Chemical
Potential
Energy
(Enthalpy)
Note that adding a catalyst
does not change the amount
of energy released (AH) for
the reaction.
Activation Energy (AE)
with a catalyst
PE of Reactants
AH
PE of Products
Rxn Progress
What effect does a catalyst have on the stoichiometry of the reaction?
What effect does the catalyst have on the mechanism of a reaction?
AE wlo a catalyst
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning