so and 20. 20, and 20 spand 2 O sp and 20, lie in different planes and do not form a bond O 20, and 20, point toward each other along the bond axis and form a e bond O sp and 2p, point towardeach other along the bond axis and formabond O seand 2p, are in the same plane and overlao side-by-side to formas bond O 20, and 20, lie in different planes and do not formabond O sp and 2p, are in the same plane and overlap side-bry-side to form a bond O sp and 2n, do not point toward each other and do not form a bond O 2n, and 2n, point toward each other along the bond axis and form a e bond O sp and 2p, point toward each other along the bond axis and forma e bond O spand 2p, point toward each other along the bond axis and formae bond plane and overlap side-by side to form a bond O sp and 20. are in the same plane and overtapside-by-side to form a e bond 20, and 20 O 2p, and 2p: point toward each other and do not form a bond O 2p, and 2p, lie indifferent planes and do not form a bond O 20, and 2p, are in the same plane and do not forma bond O 2n, and 2p, point toward each other along the bond axis and forma o bond
so and 20. 20, and 20 spand 2 O sp and 20, lie in different planes and do not form a bond O 20, and 20, point toward each other along the bond axis and form a e bond O sp and 2p, point towardeach other along the bond axis and formabond O seand 2p, are in the same plane and overlao side-by-side to formas bond O 20, and 20, lie in different planes and do not formabond O sp and 2p, are in the same plane and overlap side-bry-side to form a bond O sp and 2n, do not point toward each other and do not form a bond O 2n, and 2n, point toward each other along the bond axis and form a e bond O sp and 2p, point toward each other along the bond axis and forma e bond O spand 2p, point toward each other along the bond axis and formae bond plane and overlap side-by side to form a bond O sp and 20. are in the same plane and overtapside-by-side to form a e bond 20, and 20 O 2p, and 2p: point toward each other and do not form a bond O 2p, and 2p, lie indifferent planes and do not form a bond O 20, and 2p, are in the same plane and do not forma bond O 2n, and 2p, point toward each other along the bond axis and forma o bond
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 57E: Use Figs. 4-54 and 4-55 to answer the following questions. a. Would the bonding molecular orbital in...
Related questions
Question
100%
Decide if the following pairs of orbitals overlap to form a σ bond, π bond, or no bond at all. Choose the correct variant of explanation, including a sketch of the orbitals. Assume the bond lies along the z-axis.
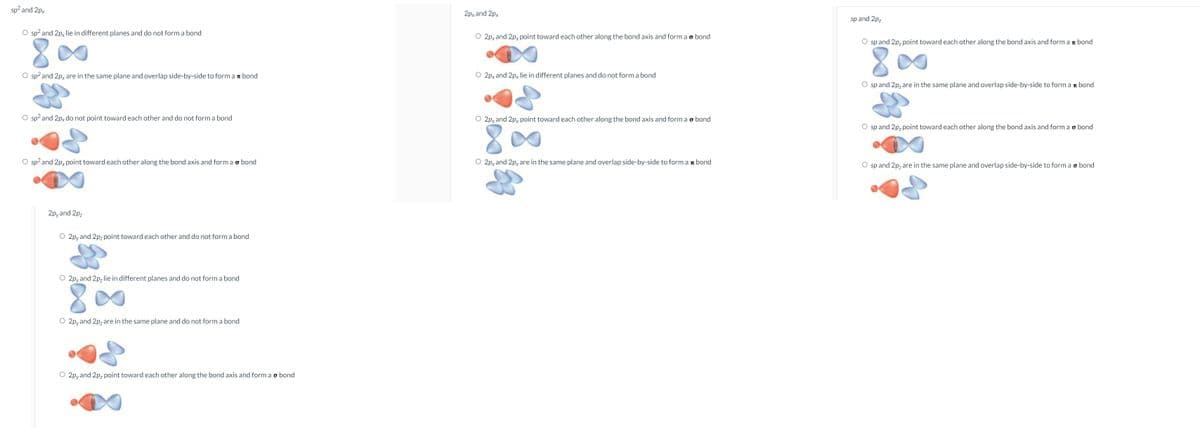
Transcribed Image Text:sp? and 2px
2px and 2px
sp and 2p,
O sp? and 2p, lie in different planes and do not form a bond
O 2py and 2px point toward each other along the bond axis and form a o bond
O sp and 2p, point toward each other along the bond axis and form a r bond
O sp? and 2px are in the same plane and overlap side-by-side to form a bond
O 2py and 2px lie in different planes and do not form a bond
O sp and 2p, are in the same plane and overlap side-by-side to form a r bond
O sp? and 2px do not point toward each other and do not form a bond
O 2pyand 2px point toward each other along the bond axis and form a o bond
O sp and 2p, point toward each other along the bond axis and form a o bond
O sp? and 2px point toward each other along the bond axis and form a o bond
O 2px and 2pyare in the same plane and overlap side-by-side to form a n bond
O sp and 2p, are in the same plane and overlap side-by-side to form a o bond
2pyand 2pz
O 2pyand 2pz point toward each other and do not form a bond
O 2pyand 2p, lie in different planes and do not form a bond
8.
O 2pyand 2pz are in the same plane and do not form a bond
O 2pyand 2pz point toward each other along the bond axis and form ao bond
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
