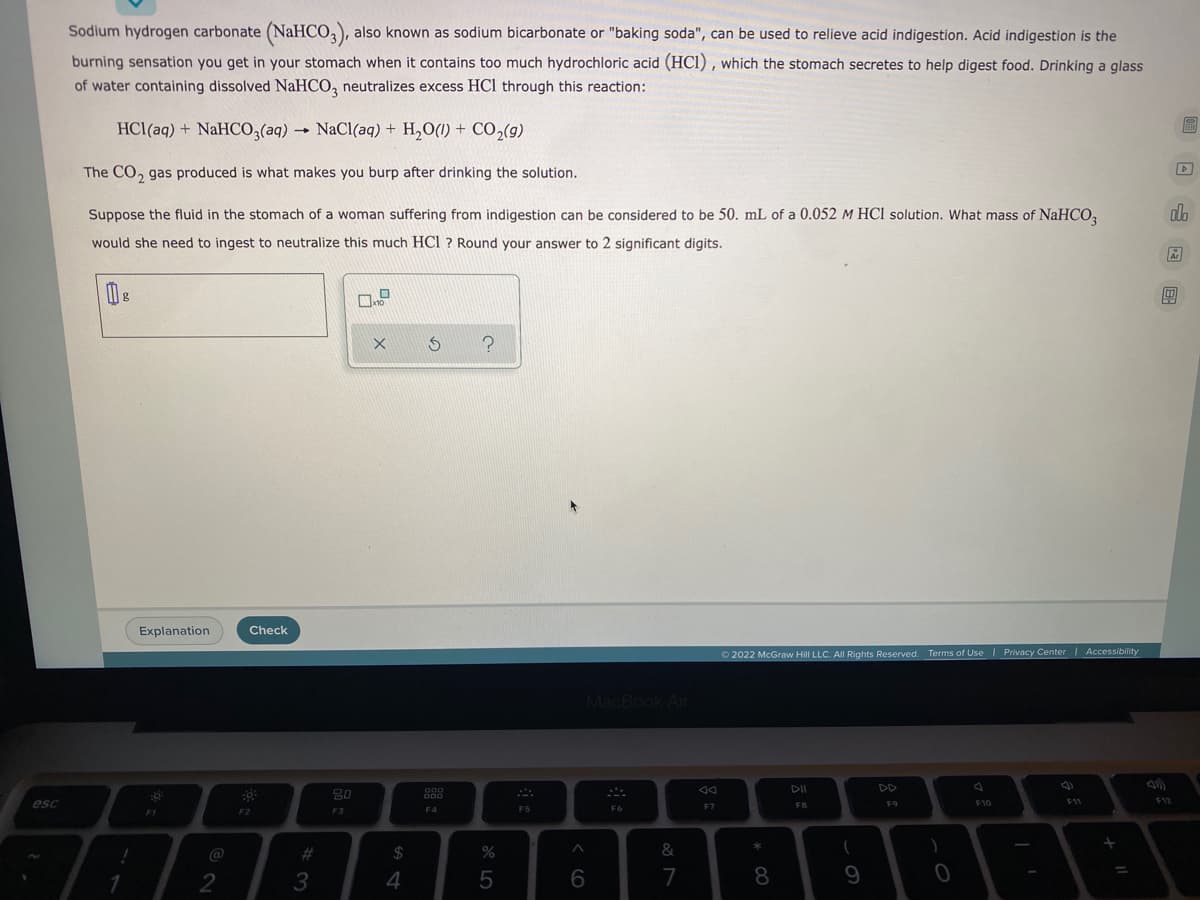
Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: 6 HCl(aq) + NaHCO3(aq) → NaCl(aq) + H₂O(l) + CO₂(g) The CO2 gas produced is what makes you burp after drinking the solution. olo Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 50. mL of a 0.052 M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits. Ś ? Å /x
Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: 6 HCl(aq) + NaHCO3(aq) → NaCl(aq) + H₂O(l) + CO₂(g) The CO2 gas produced is what makes you burp after drinking the solution. olo Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 50. mL of a 0.052 M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits. Ś ? Å /x
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 100E: Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a...
Related questions
Question
Transcribed Image Text:esc
Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the
burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass
of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction:
BEN
i
HCl(aq) + NaHCO3(aq) → NaCl(aq) + H₂O(l) + CO₂(g)
-
The CO₂ gas produced is what makes you burp after drinking the solution.
olo
Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 50. mL of a 0.052 M HCl solution. What mass of NaHCO3
would she need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits.
Ar
g
5
?
Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
DII
F11
F10
F12
FB
!
1
Explanation
F1
2
Check
F2
#
3
20
F3
%/x
$
4
000
000
F4
%
5
F5
^
6
MacBook Air
F6
&
7
F7
8
(
9
A
F9
0
8:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning