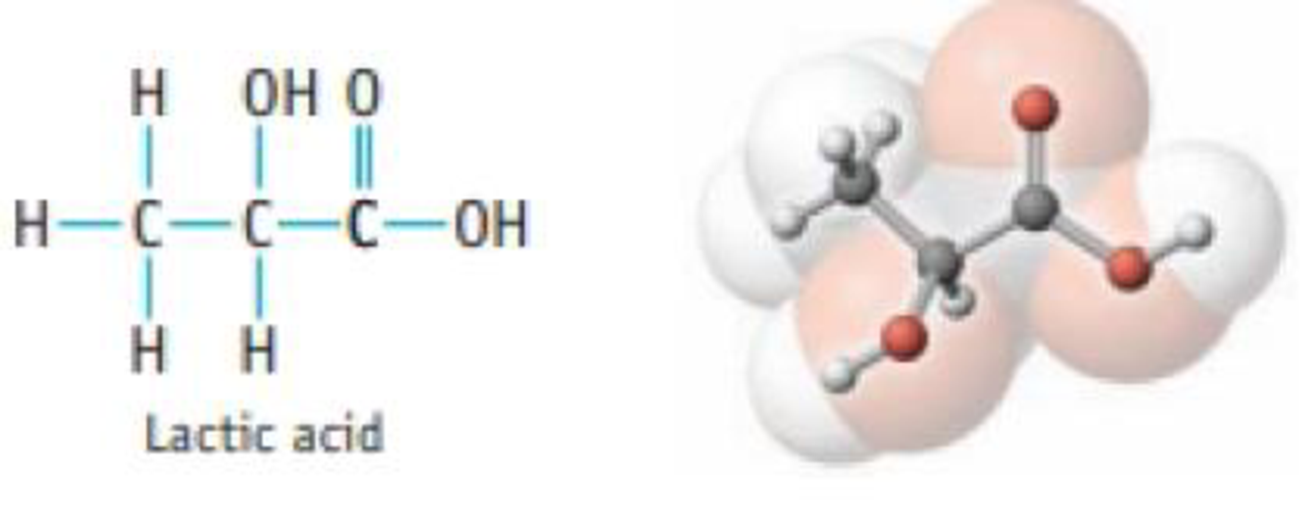
Problem 1PS: Write balanced chemical equations for the following reactions. (a) The reaction of aluminum and... Problem 2PS: Write balanced chemical equations for the following reactions: (a) production of ammonia, NH3(g), by... Problem 3PS Problem 4PS Problem 5PS Problem 6PS: Balance the following equations, and name each reactant and product: (a) SF4(g) + H2O() SO2(g) +... Problem 7PS: Equal amounts of two acidsHCl and HCO2H (formic acid)are placed in aqueous solution. When... Problem 8PS Problem 9PS: What is an electrolyte? How can you differentiate experimentally between a weak electrolyte and a... Problem 10PS: Name and give the formulas of two acids that are strong electrolytes and one acid that is a weak... Problem 11PS: Which compound or compounds in each of the following groups is (are) soluble in water? (a) CuO,... Problem 12PS: Which compound or compounds in each of the following groups is (are) soluble in water? (a) BaSO4,... Problem 13PS: The following compounds are water-soluble. What ions are produced by each compound in aqueous... Problem 14PS: The following compounds are water-soluble. What ions are produced by each compound in aqueous... Problem 15PS: Decide whether each of the following is water-soluble. If soluble, tell what ions are produced when... Problem 16PS: Decide whether each of the following is water-soluble. If soluble, tell what ions are produced when... Problem 17PS: Balance the equation for the following precipitation reaction, and then write the net ionic... Problem 18PS: Balance the equation for the following precipitation reaction, and then write the net ionic... Problem 19PS: Predict the products of each precipitation reaction. Balance the equation, and then write the net... Problem 20PS Problem 21PS: Write a balanced equation for the ionization of nitric acid in water. Problem 22PS: Write a balanced equation for the ionization of perchloric acid in water. Problem 23PS Problem 24PS: Phosphoric add can supply one, two, or three H3O+ ions in aqueous solution. Write balanced equations... Problem 25PS Problem 26PS Problem 27PS Problem 28PS Problem 29PS Problem 30PS Problem 31PS: Write an equation that describes the equilibrium that exists when nitric acid dissolves in water.... Problem 32PS: Write an equation that describes the equilibrium that exists when the weak acid benzoic acid... Problem 33PS Problem 34PS: Write two chemical equations, one in which H2PO4 is a Brnsted acid (in reaction with the carbonate... Problem 35PS: Balance the following equations, and then write the net ionic equation. (a) (NH4)2CO3(aq) +... Problem 36PS: Balance the following equations, and then write the net ionic equation: (a) Zn(s) + HCl(aq) H2(g) +... Problem 37PS Problem 38PS: Balance each of the following equations, and then write the net ionic equation. Show states for all... Problem 39PS: Write balanced net ionic equations for the following reactions: (a) the reaction of nitrous add (a... Problem 40PS: Write balanced net ionic equations for the following reactions: (a) the reaction of aqueous... Problem 41PS: Siderite is a mineral consisting largely of iron(II) carbonate. Write an overall, balanced equation... Problem 42PS: The mineral rhodothrosite is manganese() carbonate. Write an overall, balanced equation for the... Problem 43PS Problem 44PS Problem 45PS: Determine the oxidation number of each element in the following ions or compounds. (a) BrO3- (b)... Problem 46PS: Determine the oxidation number of each element in the following ions or compounds. (a) PF6 (b)... Problem 47PS Problem 48PS: Which two of the following reactions are oxidation-reduction reactions? Explain your answer briefly.... Problem 49PS: In the following reactions, decide which reactant is oxidized and which is reduced. Designate the... Problem 50PS: In the following reactions, decide which reactant is oxidized and which is reduced. Designate the... Problem 51PS: Balance the following equations, and then classify each as a precipitation, acid-base, or... Problem 52PS Problem 53PS: Classify each of the following reactions as a precipitation, acid-base, or gas forming reaction.... Problem 54PS Problem 55PS: Balance each of the following equations, and classify them as precipitation, acid-base, gas-forming,... Problem 56PS: Complete and balance the equations below, and classify them as precipitation, acid-base,... Problem 57PS Problem 58PS Problem 59GQ: Balance the following equations: (a) for the synthesis of urea, a common fertilizer CO2(g) + NH3(g) ... Problem 60GQ: Balance the following equations: (a) for the reaction to produce "superphosphate" fertilizer... Problem 61GQ Problem 62GQ: Give the formula for each of the following compounds: (a) a soluble compound containing the acetate... Problem 63GQ Problem 64GQ: Name two anions that combine with Al3+ ion to produce water-soluble compounds. Problem 65GQ: Write the net ionic equation and identify the spectator ion or ions in the reaction of nitric acid... Problem 66GQ: Identify and name the water-insoluble product in each reaction and write the net ionic equation: (a)... Problem 67GQ: Bromine is obtained from sea water by the following redox reaction: Cl2(g) + 2 NaBr(aq) 2 NaCl(aq)... Problem 68GQ: Identify each of the blowing substances as a likely oxidizing or reducing agent: HNO3, Na, C12, O2,... Problem 69GQ: The mineral dolomite contains magnesium carbon-ate. This reacts with hydrochloric add. MgCO3(s) + 2... Problem 70GQ: Aqueous solutions of ammonium sulfide, (NH4)2S, and Hg(NO3)2 react to produce HgS and NH4NO3. (a)... Problem 71GQ Problem 72GQ Problem 73GQ: Balance equations for these reactions that occur in aqueous solution, and then classify each as a... Problem 74GQ Problem 75GQ: You are given mixtures containing the following compounds. Which compound in each pair could be... Problem 76GQ: Identify, from each list below, the compound or compounds that will dissolve in water to give a... Problem 77GQ Problem 78GQ Problem 79GQ: Gas evolution was observed when a solution of Na2S was treated with acid. The gas was bubbled into a... Problem 81IL Problem 82IL Problem 83IL Problem 84IL: A Suggest a laboratory method for preparing barium phosphate. (See Study Question 97 for a way to... Problem 85IL: The Toliens test for the presence of reducing sugars (say, in a urine sample) involves treating the... Problem 86SCQ: There are many ionic compounds that dissolve in water to a very small extent. One example is... Problem 87SCQ: Most naturally occurring acids are weak acids. Lactic acid is one example.... Problem 88SCQ: You want to prepare barium chloride, BaC12, using an exchange reaction of some type. To do so, you... Problem 89SCQ Problem 90SCQ: A Describe how to prepare zinc chloride by (a) an add-base reaction, (b) a gas-forming reaction, and... Problem 91SCQ: A common method for analyzing for the nickel content of a sample is to use a precipitation reaction.... Problem 93SCQ: The presence of arsenic in a sample that may also contain another Group 5A element, antimony, can be... format_list_bulleted


 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
