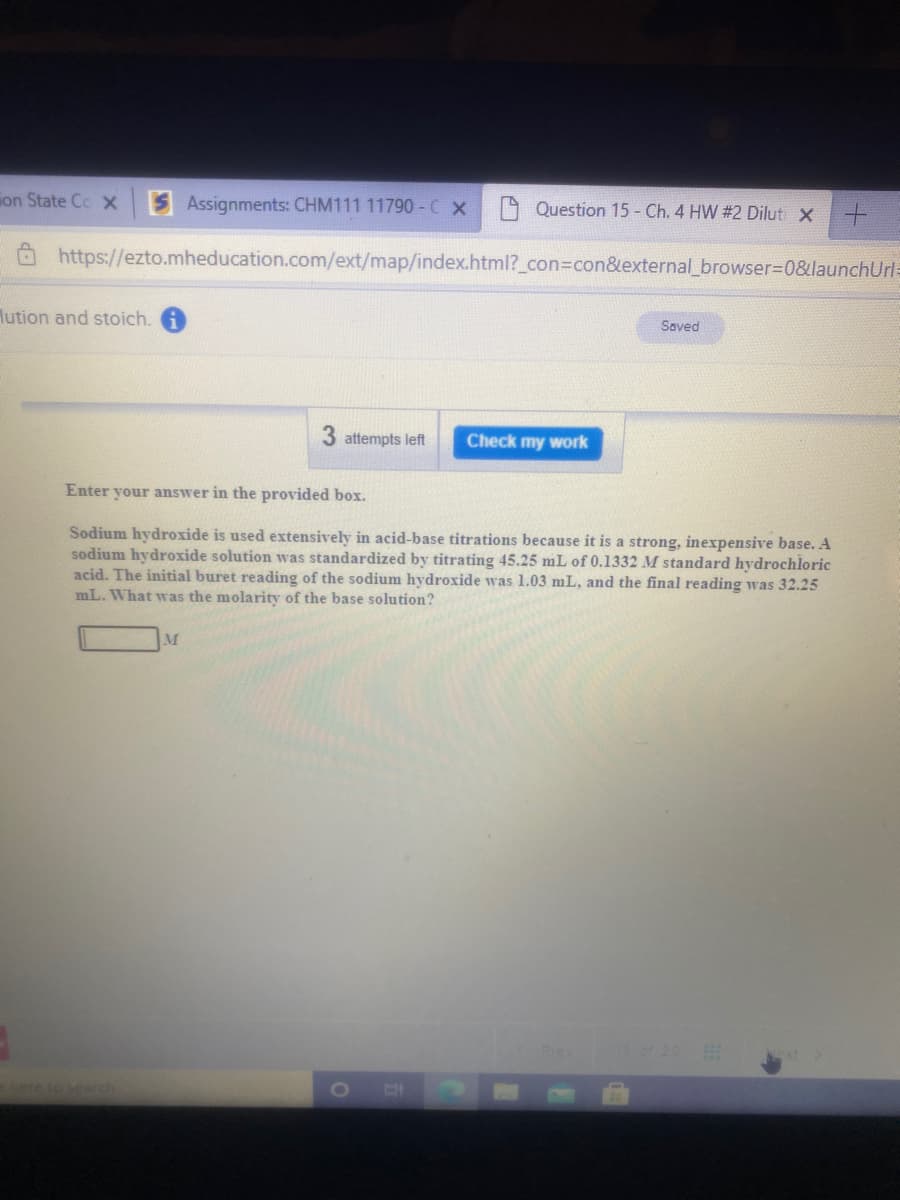
Sodium hydroxide is used extensively in acid-base titrations because it is a strong, inexpensive base. A sodium hydroxide solution was standardized by titrating 45.25 mL of 0.1332 M standard hydrochloric acid. The initial buret reading of the sodium hydroxide was 1.03 mL, and the final reading was 32.25 mL. What was the molarity of the base solution? M
Sodium hydroxide is used extensively in acid-base titrations because it is a strong, inexpensive base. A sodium hydroxide solution was standardized by titrating 45.25 mL of 0.1332 M standard hydrochloric acid. The initial buret reading of the sodium hydroxide was 1.03 mL, and the final reading was 32.25 mL. What was the molarity of the base solution? M
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter12: Atomic X-ray Spectrometry
Section: Chapter Questions
Problem 12.10QAP
Related questions
Question
Transcribed Image Text:ion State Cc X
Assignments: CHM111 11790- C x
Question 15 - Ch. 4 HW #2 Dilut x +
https://ezto.mheducation.com/ext/map/index.html?_con%3Dcon&external_browser3D0&launchUrl:
lution and stoich.
Saved
attempts left
Check my work
Enter your answer in the provided box.
Sodium hydroxide is used extensively in acid-base titrations because it is a strong, inexpensive base. A
sodium hydroxide solution was standardized by titrating 45.25 mL of 0.1332 M standard hydrochloric
acid. The initial buret reading of the sodium hydroxide was 1.03 mL, and the final reading was 32.25
mL. What was the molarity of the base solution?
search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning