Solid sodium chromate is slowly added to 150 mL of a 0.0675 M lead nitrate solution. The concentration of chromate ion required to just initiate precipitation is M.
Solid sodium chromate is slowly added to 150 mL of a 0.0675 M lead nitrate solution. The concentration of chromate ion required to just initiate precipitation is M.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter15: Complex Ion And Precipitation Equilibria
Section: Chapter Questions
Problem 30QAP: Silver(I) sulfate (Ksp=1.2105) is used in the electroplating of silver. A 1.0-L solution is prepared...
Related questions
Question
100%
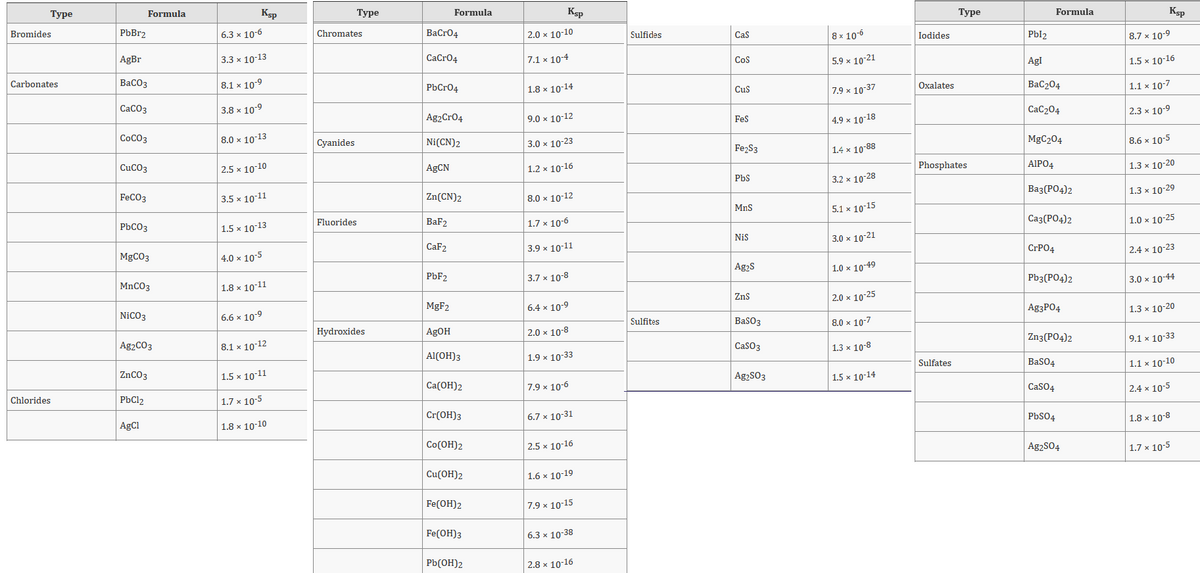
Transcribed Image Text:Туре
Ksp
Туре
Formula
Ksp
Туре
Formula
Ksp
Formula
Bromides
PbBr2
6.3 x 10-6
Chromates
ВаCr04
2.0 x 10-10
8 x 10-6
Pbl2
8.7 x 10-9
Sulfides
Cas
Iodides
AgBr
3.3 x 10-13
CaCro4
7.1 x 104
5.9 x 10-21
Cos
Agl
1.5 x 10-16
Carbonates
ВаСОз
8.1 x 10-9
BaC204
1.1 x 10-7
PbCr04
1.8 x 10-14
7.9 x 10-37
Oxalates
Cus
СаСОз
3.8 x 10-9
СаС204
2.3 x 10-9
Ag2Cr04
9.0 x 10-12
Fes
| 4.9 × 10-18
CoCO3
8.0 x 10-13
Cyanides
Ni(CN)2
3.0 x 10-23
MgC204
8.6 x 10-5
Fe2S3
1.4 x 10-88
CUCO3
| 2.5 x 10-10
| AGCN
1.2 x 10-16
Phosphates
AIPO4
1.3 x 10-20
PbS
3.2 x 10-28
|Ваз (РО4)2
1.3 x 10-29
FeCO3
3.5 x 10-11
Zn(CN)2
8.0 x 10-12
MnS
5.1 x 10-15
Fluorides
BaF2
| 1.7 x 10-6
Саз(РО4)2
1.0 x 10-25
PBCO3
| 1.5 x 10-13
Nis
3.0 x 10-21
CaF2
3.9 x 10-11
CIPO4
| 2.4 x 10-23
MGCO3
| 4.0 × 10-5
Ag2S
1.0 x 10-49
PBF2
3.7 x 10-8
Pb3(PO4)2
3.0 x 10-44
MNCO3
1.8 x 10-11
Zns
2.0 x 10-25
MGF2
6.4 x 10-9
Ag3PO4
1.3 x 10-20
NiCO3
6.6 x 10-9
Sulfites
Baso3
8.0 × 10-7
Hydroxides
AgOH
|2.0 x 10-8
Zn3(PO4)2
9.1 x 10-33
Ag2CO3
8.1 x 10-12
Caso3
1.3 x 10-8
Al(OH)3
1.9 x 10-33
Sulfates
BaSO4
1.1 x 10-10
ZNCO3
| 1.5 x 10-11
AgąS03
1.5 x 10-14
Ca(OH)2
7.9 x 10-6
CaSO4
|2.4 x 10-5
Chlorides
PbCl2
1.7 x 10-5
Cr(ОH)з
6.7 x 10-31
PbS04
1.8 x 10-8
AgCl
1.8 x 10-10
Cо(ОН)2
| 2.5 x 10-16
Ag2S04
1.7 x 10-5
Cu(OH)2
1.6 х 10-19
Fe(ОН)2
7.9 x 10-15
Fe(ОH)з
6.3 х 10-38
Pb(OH)2
2.8 x 10-16
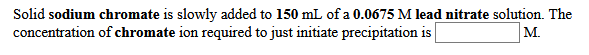
Transcribed Image Text:Solid sodium chromate is slowly added to 150 mL of a 0.0675 M lead nitrate solution. The
concentration of chromate ion required to just initiate precipitation is
M.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning