Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by three quantum numbers: n, 1, and m. If the value of n= 3 The quantum number I can have values from to The total number of orbitals possible at the n = 3 energy level is If the value of I = 2 The quantum number m, can have values from The total number of orbitals possible at the 1= 2 sublevel is to
Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by three quantum numbers: n, 1, and m. If the value of n= 3 The quantum number I can have values from to The total number of orbitals possible at the n = 3 energy level is If the value of I = 2 The quantum number m, can have values from The total number of orbitals possible at the 1= 2 sublevel is to
Modern Physics
3rd Edition
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Chapter8: Quantum Mechanics In Three Dimensions
Section: Chapter Questions
Problem 12P
Related questions
Question
answer the given picture and do not round off until the final answer. thank you
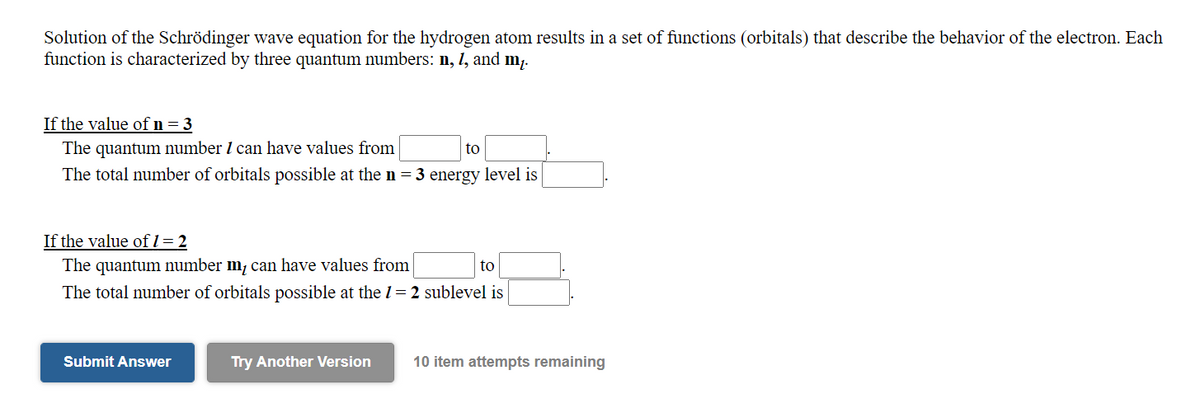
Transcribed Image Text:Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each
function is characterized by three quantum numbers: n, l, and m,.
If the value of n= 3
The quantum number I can have values from
The total number of orbitals possible at the n = 3 energy level is
to
If the value of l= 2
The quantum number m, can have values from
to
The total number of orbitals possible at the 1= 2 sublevel is
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning