Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by three quantum numbers: n, I, and m,. If the value of n = 3 The quantum number I can have values from The total number of orbitals possible at the n = 3 energy level is If the value of 1 = 1 The quantum number m, can have values from to The total number of orbitals possible at the I = 1 sublevel is
Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. Each function is characterized by three quantum numbers: n, I, and m,. If the value of n = 3 The quantum number I can have values from The total number of orbitals possible at the n = 3 energy level is If the value of 1 = 1 The quantum number m, can have values from to The total number of orbitals possible at the I = 1 sublevel is
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 59AP
Related questions
Question
Solve correctly please, need all 4 subparts ans.
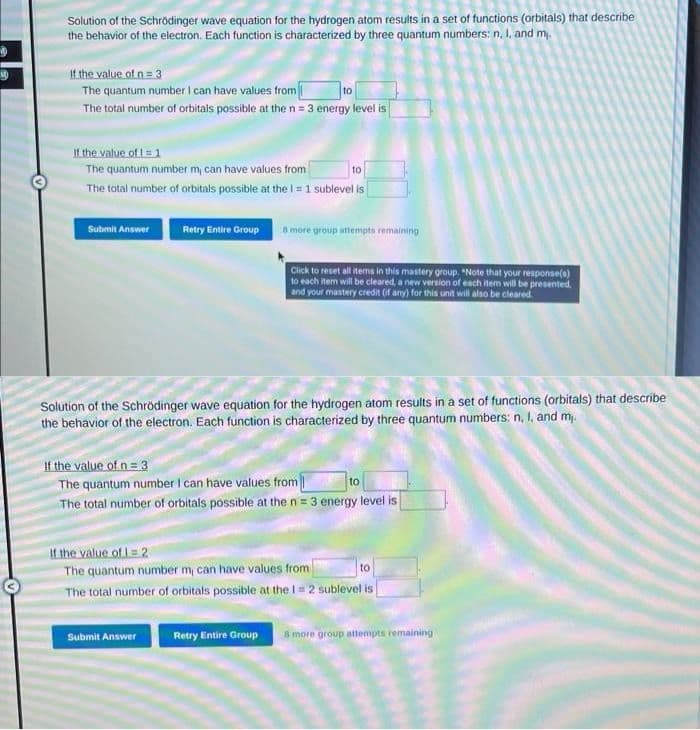
Transcribed Image Text:Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe
the behavior of the electron. Each function is characterized by three quantum numbers: n, I, and my.
If the value of n = 3
The quantum number I can have values from
to
The total number of orbitals possible at the n = 3 energy level is
If the value of 1 = 1
The quantum number m, can have values from
to
The total number of orbitals possible at the I = 1 sublevel is
Submit Answer
Retry Entire Group
8 more group attempts remaining
Click to reset all items in this mastery group. "Note that your response(s)
to each item will be cleared, a new version of each item will be presented.
and your mastery credit (if any) for this unit will also be cleared.
Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe
the behavior of the electron. Each function is characterized by three quantum numbers: n, I, and m,.
Submit Answer
If the value of n = 3
The quantum number I can have values from
The total number of orbitals possible at the n = 3 energy level is
to
If the value of 1-2
The quantum number m, can have values from
to
The total number of orbitals possible at the 1 = 2 sublevel is
Retry Entire Group 8 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,