Solution pH 1 pH 3 Alizarin Yellow yellow colourles Thymol Bromothym Bromocreso I Green Blue ol Blue (Magenta Indicator Yellow UOP yellow Yellow yellow orange Yellow Methyl Orange Red Red- orange Red Cabbage Juice pink Purple
Solution pH 1 pH 3 Alizarin Yellow yellow colourles Thymol Bromothym Bromocreso I Green Blue ol Blue (Magenta Indicator Yellow UOP yellow Yellow yellow orange Yellow Methyl Orange Red Red- orange Red Cabbage Juice pink Purple
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 99AP
Related questions
Question
please explain to me how to answer the "analysis" question.
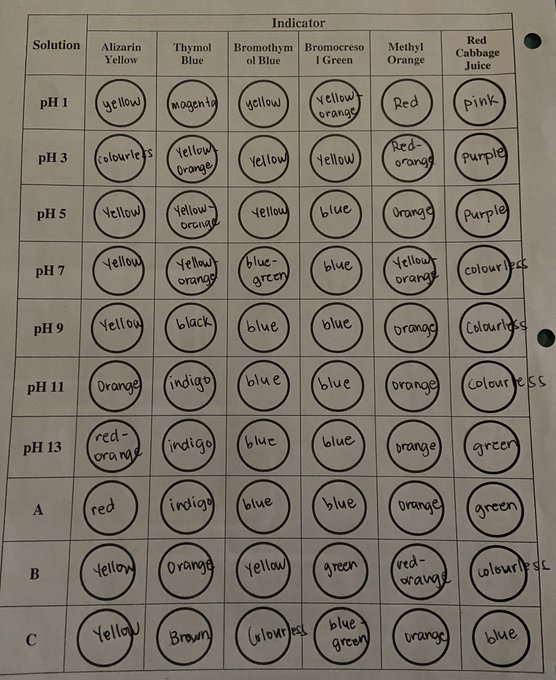
Transcribed Image Text:Solution
pH 1
pH 3
pH 5
pH 7
pH 9
pH 11
pH 13
A
B
C
Alizarin
Yellow
yellow
colourlers
Yellow
Yellow
Yellow
Drange
red-
orange
red
Yellow
Yellow
Thymol Bromothym Bromocreso
Blue
ol Blue
I Green
magenta
Yellow
Orange
Yellow
orange
Yellow
orange
black
indigo
(indigo)
indigo
Orange
Indicator
Brown
yellow
Yellow
Yellow
blue-
green
blue
blue
blue
blue
Yellow
Colourless
yellow
orange
Yellow
blue
blue
blue
blue
blue
blue
green
blue
greeny
Methyl
Orange
Red
Red-
orange
Orange
Yellow
orange
orange
(orange)
orange
orange
ved-
orange
orange
Red
Cabbage
Juice
Pink
Purple
Purple
colourless
Colourless
Colourless
green
green
colourless
blue

Transcribed Image Text:Purpose: You will demonstrate the use of indicators to determine the pH of a solution.
Variables:
Manipulated Variable: Type of solution.
Responding Variable: indicator
Controlled Variables:
Observations: see other side
Analysis: For each unknown solution, A, B & C, determine the range of its pH and explain how you used the
indicators to determine the pH range for each unknown solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

