solution was prepared by dissolving 34.7 g of KOH in 88.0 g of ethanol (C₂H5OH). A What is the molality of the solution? B. What is the van't Hoff factor (i) and colligative molality for the solution? c. What is the boiling point of the solution? Show all work on separate paper and upload as a PDF with the rest of your exam work. Please circle all final answers. In the answer space provided, please write "see attached." For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIVS Paragraph V Arial SE 田出田园田 V <> (0) 10pt X² X₂ & Be V Av 2 IX ✓ 11 "152 *** B
solution was prepared by dissolving 34.7 g of KOH in 88.0 g of ethanol (C₂H5OH). A What is the molality of the solution? B. What is the van't Hoff factor (i) and colligative molality for the solution? c. What is the boiling point of the solution? Show all work on separate paper and upload as a PDF with the rest of your exam work. Please circle all final answers. In the answer space provided, please write "see attached." For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIVS Paragraph V Arial SE 田出田园田 V <> (0) 10pt X² X₂ & Be V Av 2 IX ✓ 11 "152 *** B
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter10: Properties Of Solutions
Section: Chapter Questions
Problem 120CP: Plants that thrive in salt water must have internal solutions (inside the plant cells) that are...
Related questions
Question
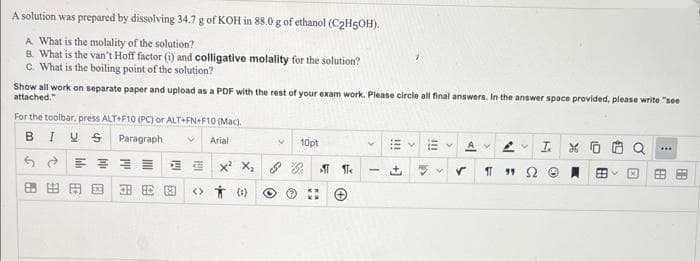
Transcribed Image Text:A solution was prepared by dissolving 34.7 g of KOH in 88.0 g of ethanol (C2H5OH).
A What is the molality of the solution?
B. What is the van't Hoff factor (i) and colligative molality for the solution?
C. What is the boiling point of the solution?
Show all work on separate paper and upload as a PDF with the rest of your exam work. Please circle all final answers. In the answer space provided, please write "see
attached."
For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac).
BIUS
Paragraph
V Arial
6 ते
E
FA
10pt
EX¹ X₂ & B¶¶<
奥园田园 <> 0)
V
Αν Αν τα
✓
11 99
Ⓒ
B
F
***
F
88
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning