11. Several conformational isomers of butane are illustrated below. Which has the LOWEST energy? CH3 (A) (B) (C) (D) (E) H H I CH3 H I CH3 H H. H II III IV they are all equal in energy H II H CH3 HCH3 H H₂C III -H H H3CCH3 H H IV -H H
11. Several conformational isomers of butane are illustrated below. Which has the LOWEST energy? CH3 (A) (B) (C) (D) (E) H H I CH3 H I CH3 H H. H II III IV they are all equal in energy H II H CH3 HCH3 H H₂C III -H H H3CCH3 H H IV -H H
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter20: Chemistry Of Hydrogen, Elements In Group 3a Through 6a, And The Noble Gases
Section: Chapter Questions
Problem 20.24QE
Related questions
Question
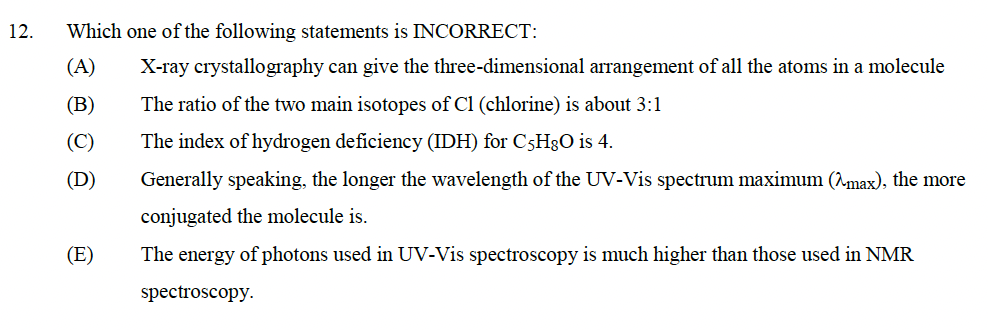
Transcribed Image Text:12.
Which one of the following statements is INCORRECT:
(A)
X-ray crystallography can give the three-dimensional arrangement of all the atoms in a molecule
The ratio of the two main isotopes of Cl (chlorine) is about 3:1
(B)
(C)
The index of hydrogen deficiency (IDH) for C5H8O is 4.
(D)
Generally speaking, the longer the wavelength of the UV-Vis spectrum maximum (2max), the more
conjugated the molecule is.
The energy of photons used in UV-Vis spectroscopy is much higher than those used in NMR
spectroscopy.
(E)
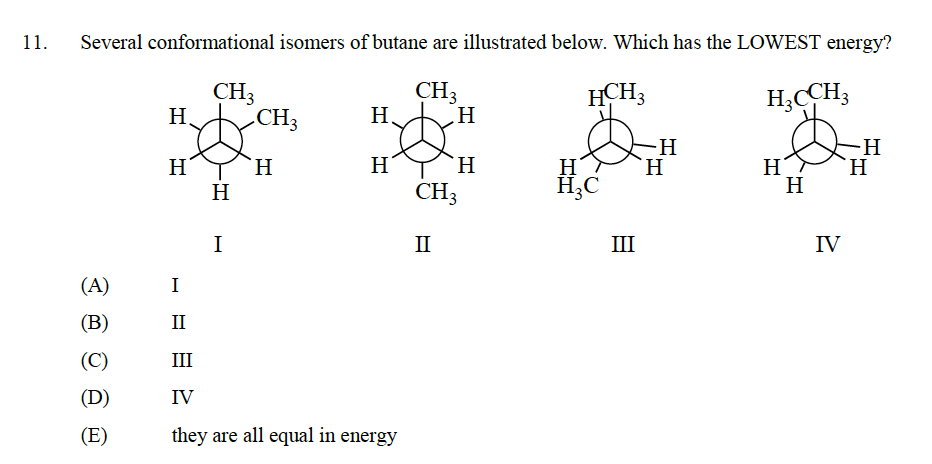
Transcribed Image Text:11.
Several conformational isomers of butane are illustrated below. Which has the LOWEST energy?
CH3
HCH3
H3CCH3
(A)
(B)
(C)
(D)
(E)
Η.
H
I
CH3
HI
Η
I
CH3
H
H
H
II
III
IV
they are all equal in energy
H
H
CH3
II
H
H₂C
III
H
H
H
H
IV
-H
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning