Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter8: Addition Via Carbocation
Section: Chapter Questions
Problem 8E
Related questions
Question
Solve all parts otherwise I will downvote
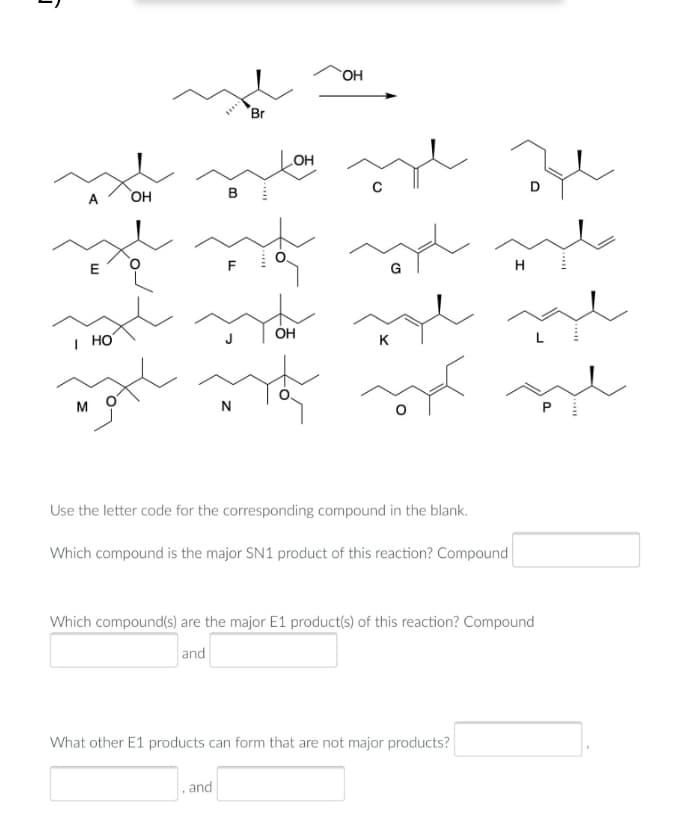
Transcribed Image Text:OH
Br
HO
A
OH
E
H
| HO
ÓH
K
M
N
Use the letter code for the corresponding compound in the blank.
Which compound is the major SN1 product of this reaction? Compound
Which compound(s) are the major E1 product(s) of this reaction? Compound
and
What other E1 products can form that are not major products?
and
....
B.
![Assign if each of these statements are true or false:
[ Select ]
SN1 and E1 have the same rate law.
[ Select ]
We can get SN1 reactions without any E1 side product.
[ Select ]
We can get E1 reactions without any SN1 side product.
[ Select ]
Increasing the amount of good nucleophile increases the ratio
of SN1:E1 product.
[ Select ]
E1 reactions make alkenes at any carbon in the haloalkane.
[ Select ]
Trans alkenes are more stable than cis alkenes.
[ Select ]
E1 reactions prefer to form the most substituted alkene
because it's most stable.
[ Select ]
Unimolecular mechanisms go through a chiral transition state
and have predictable stereochemistry in the products.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F120ef40c-ba61-489a-8f0e-beaff5d51a86%2F46272c5a-fb90-4f3c-b953-21be9e0ae9fb%2F2876j5y_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Assign if each of these statements are true or false:
[ Select ]
SN1 and E1 have the same rate law.
[ Select ]
We can get SN1 reactions without any E1 side product.
[ Select ]
We can get E1 reactions without any SN1 side product.
[ Select ]
Increasing the amount of good nucleophile increases the ratio
of SN1:E1 product.
[ Select ]
E1 reactions make alkenes at any carbon in the haloalkane.
[ Select ]
Trans alkenes are more stable than cis alkenes.
[ Select ]
E1 reactions prefer to form the most substituted alkene
because it's most stable.
[ Select ]
Unimolecular mechanisms go through a chiral transition state
and have predictable stereochemistry in the products.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning