Some Al,Cl, (at a partial pressure of 0.473 atm) is placed in a closed container at 454 K with some Al3Cl, (at a par- tial pressure of 1.02 × 10-2 atm). Enough argon is added to raise the total pressure to 1.00 atm. (a) Calculate the initial reaction quotient for the reaction 3 Al,Cl.(g) = 2 Al;Cl,(g) (b) As the gas mixture reaches equilibrium, will there be net production or consumption of Al;Cl,? (Use the data given in problem 21.)
Some Al,Cl, (at a partial pressure of 0.473 atm) is placed in a closed container at 454 K with some Al3Cl, (at a par- tial pressure of 1.02 × 10-2 atm). Enough argon is added to raise the total pressure to 1.00 atm. (a) Calculate the initial reaction quotient for the reaction 3 Al,Cl.(g) = 2 Al;Cl,(g) (b) As the gas mixture reaches equilibrium, will there be net production or consumption of Al;Cl,? (Use the data given in problem 21.)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section12.5: Using Equilibrium Constants
Problem 12.7CE
Related questions
Question
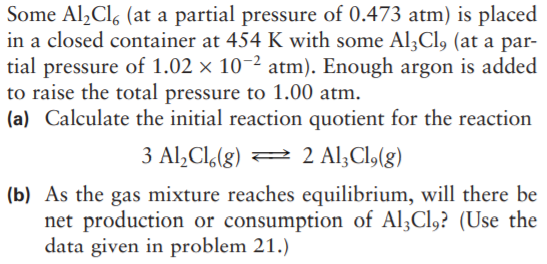
Transcribed Image Text:Some Al,Cl, (at a partial pressure of 0.473 atm) is placed
in a closed container at 454 K with some Al3Cl, (at a par-
tial pressure of 1.02 × 10-2 atm). Enough argon is added
to raise the total pressure to 1.00 atm.
(a) Calculate the initial reaction quotient for the reaction
3 Al,Cl.(g) =
2 Al;Cl,(g)
(b) As the gas mixture reaches equilibrium, will there be
net production or consumption of Al;Cl,? (Use the
data given in problem 21.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning