Some measurements of the initial rate of a certain reaction are given in the table below. N2 H2 initial rate of reaction 1.40 м 0.148м 0.0496 M/s 1.40M 0.220 M 0.0737 M/s 2.75 M 0.148 M 0.191 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k||
Some measurements of the initial rate of a certain reaction are given in the table below. N2 H2 initial rate of reaction 1.40 м 0.148м 0.0496 M/s 1.40M 0.220 M 0.0737 M/s 2.75 M 0.148 M 0.191 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k||
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 33E: The reaction I(aq)+OCl(aq)IO(aq)+Cl(aq) was studied, and the following data were obtained:...
Related questions
Question
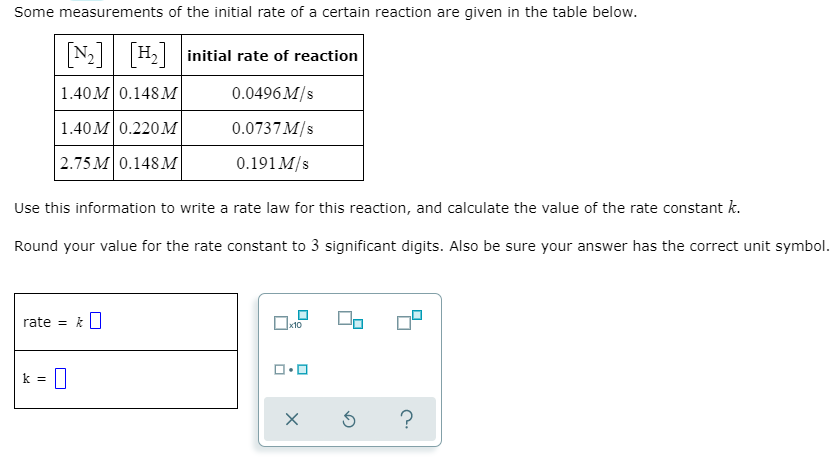
Transcribed Image Text:Some measurements of the initial rate of a certain reaction are given in the table below.
N2 H2 initial rate of reaction
1.40M0.148 M
0.0496 M/s
1.40M 0.220M
0.0737 M/s
2.75 M 0.148 M
0.191 M/s
Use this information to write a rate law for this reaction, and calculate the value of the rate constant k.
Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol.
rate = k||
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning