Spiropentane, shown on the left, reacts with Cl2 in the presence of light to furnish the two products shown on the right. Given that Cl2 is readily cleaved by light, provide a detailed step-by-step mechanism for the formation of the two products from the common intermediate shown in brackets, and also show the mechanism for the formation of the common intermediate from spiropentane. Use curved arrows to detail electron flow. Cl Cl2 + hv
Spiropentane, shown on the left, reacts with Cl2 in the presence of light to furnish the two products shown on the right. Given that Cl2 is readily cleaved by light, provide a detailed step-by-step mechanism for the formation of the two products from the common intermediate shown in brackets, and also show the mechanism for the formation of the common intermediate from spiropentane. Use curved arrows to detail electron flow. Cl Cl2 + hv
Chapter11: Reactions Of Alkyl Halides: Nucleophilic Substitutions And Eliminations
Section11.SE: Something Extra
Problem 74AP
Related questions
Question
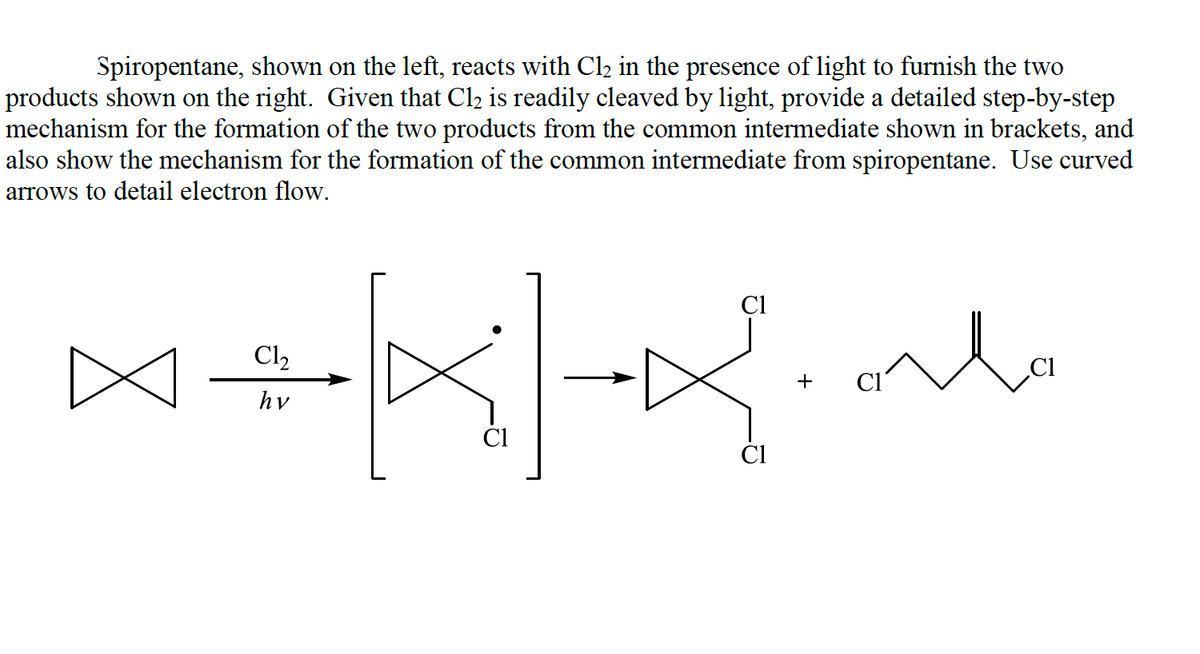
Transcribed Image Text:Spiropentane, shown on the left, reacts with Cl2 in the presence of light to furnish the two
products shown on the right. Given that Cl2 is readily cleaved by light, provide a detailed step-by-step
mechanism for the formation of the two products from the common intermediate shown in brackets, and
also show the mechanism for the formation of the common intermediate from spiropentane. Use curved
arrows to detail electron flow.
Cl
lo
Cl2
+
hv
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning