Splitting of a signal in a proton NMR spectrum tells us the number of chemically non- equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the number of lines exhibited by hydrogens at the labeled positions in a first-order NMR spectrum. (Make the approximation that all coupling constants are equal.) 1) HO. The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is 2) b NH2 The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is
Splitting of a signal in a proton NMR spectrum tells us the number of chemically non- equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the number of lines exhibited by hydrogens at the labeled positions in a first-order NMR spectrum. (Make the approximation that all coupling constants are equal.) 1) HO. The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is 2) b NH2 The number of lines exhibited by hydrogen(s) a is The number of lines exhibited by hydrogen(s) b is The number of lines exhibited by hydrogen(s) c is
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter12: Nuclear Magnetic Resonance Spectroscopy
Section: Chapter Questions
Problem 1Q
Related questions
Question
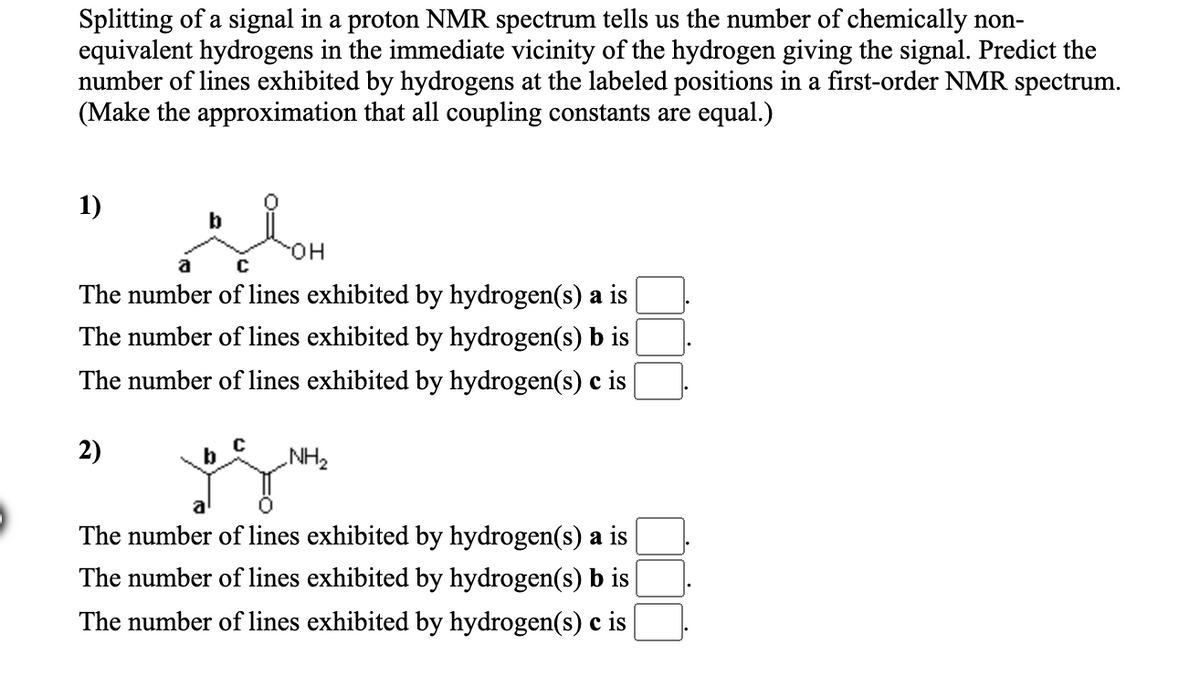
Transcribed Image Text:Splitting of a signal in a proton NMR spectrum tells us the number of chemically non-
equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the
number of lines exhibited by hydrogens at the labeled positions in a first-order NMR spectrum.
(Make the approximation that all coupling constants are equal.)
1)
HO.
a
The number of lines exhibited by hydrogen(s) a is
The number of lines exhibited by hydrogen(s) b is
The number of lines exhibited by hydrogen(s) c is
2)
b
NH2
The number of lines exhibited by hydrogen(s) a is
The number of lines exhibited by hydrogen(s) b is
The number of lines exhibited by hydrogen(s) c is
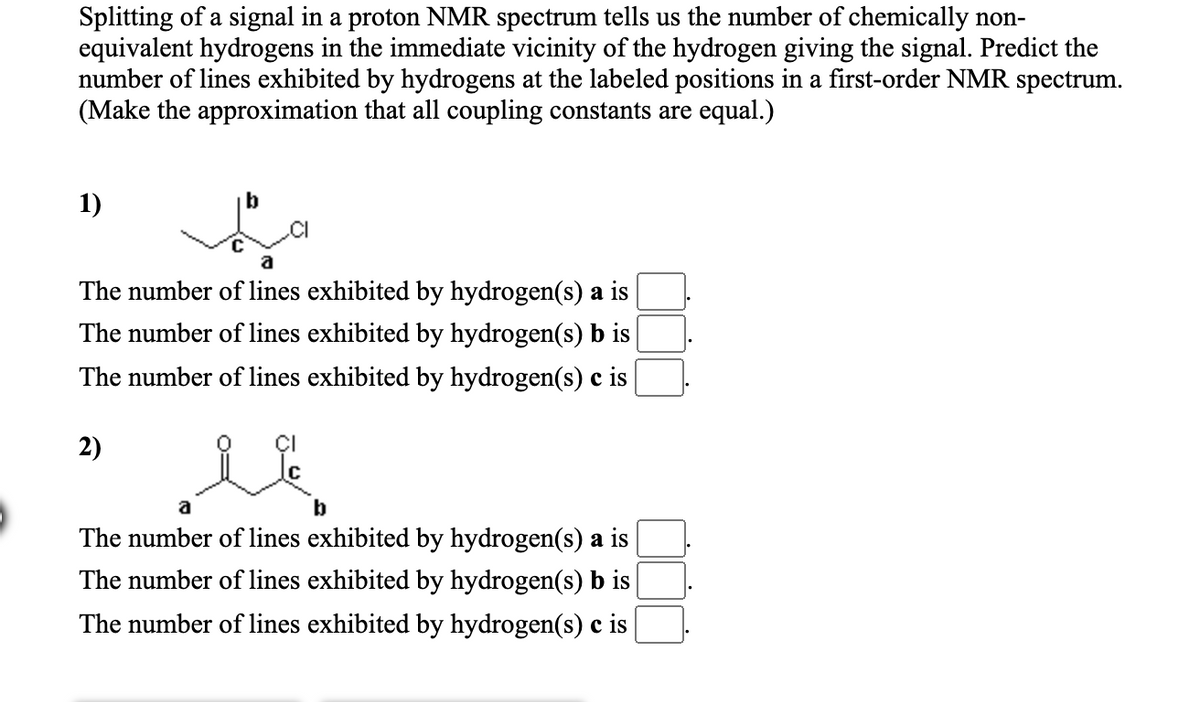
Transcribed Image Text:Splitting of a signal in a proton NMR spectrum tells us the number of chemically non-
equivalent hydrogens in the immediate vicinity of the hydrogen giving the signal. Predict the
number of lines exhibited by hydrogens at the labeled positions in a first-order NMR spectrum.
(Make the approximation that all coupling constants are equal.)
1)
.CI
The number of lines exhibited by hydrogen(s) a is
The number of lines exhibited by hydrogen(s) b is
The number of lines exhibited by hydrogen(s) c is
2)
a
b
The number of lines exhibited by hydrogen(s) a is
The number of lines exhibited by hydrogen(s) b is
The number of lines exhibited by hydrogen(s) c is
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning