ssignment > Open Assignment CALCULATOR FULL SCREEN PRINTER VERSION NEXT ( BACK SSIGNMENT RESOURCES ATALYST: c10 Kinetic Jolecular Theory- inetic Energy of Gases Question 1 Kinetic Energy of Gases T03/S05 Calculate the average kinetic energy (J/molecule) for a molecule of an ideal gas at 553 K. Gas Constant (J/Mol K) R 8.31451 Avogadro's No (mol"1) Question Question 3 Question 4 Question 5 Question 6 6.022x1023 | J/molecule the tolerance is +/-2% eview Score RESET Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
ssignment > Open Assignment CALCULATOR FULL SCREEN PRINTER VERSION NEXT ( BACK SSIGNMENT RESOURCES ATALYST: c10 Kinetic Jolecular Theory- inetic Energy of Gases Question 1 Kinetic Energy of Gases T03/S05 Calculate the average kinetic energy (J/molecule) for a molecule of an ideal gas at 553 K. Gas Constant (J/Mol K) R 8.31451 Avogadro's No (mol"1) Question Question 3 Question 4 Question 5 Question 6 6.022x1023 | J/molecule the tolerance is +/-2% eview Score RESET Question Attempts: 0 of 1 used SUBMIT ANSWER SAVE FOR LATER
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter8: Properties Of Gases
Section: Chapter Questions
Problem 69QRT
Related questions
Question
please help me
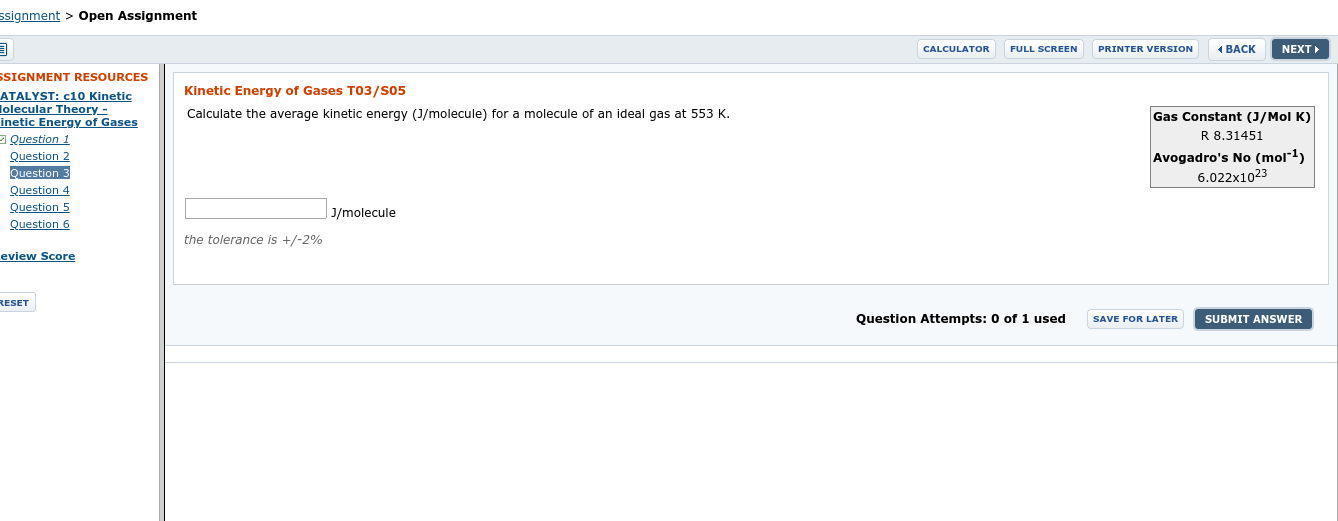
Transcribed Image Text:ssignment > Open Assignment
CALCULATOR
FULL SCREEN
PRINTER VERSION
NEXT
( BACK
SSIGNMENT RESOURCES
ATALYST: c10 Kinetic
Jolecular Theory-
inetic Energy of Gases
Question 1
Kinetic Energy of Gases T03/S05
Calculate the average kinetic energy (J/molecule) for a molecule of an ideal gas at 553 K.
Gas Constant (J/Mol K)
R 8.31451
Avogadro's No (mol"1)
Question
Question 3
Question 4
Question 5
Question 6
6.022x1023
| J/molecule
the tolerance is +/-2%
eview Score
RESET
Question Attempts: 0 of 1 used
SUBMIT ANSWER
SAVE FOR LATER
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
