Pf = P;/2 A sample of an ideal gas at P, is initially confined to one chamber of the apparatus represented above, and the other chamber is initially evacuated. The valve connecting the chambers is opened, and the gas expands at constant temperature to fill both chambers. Which of the following best describes AS for the process? A AS 0 because the gas particles become dispersed in a larger volume.
Pf = P;/2 A sample of an ideal gas at P, is initially confined to one chamber of the apparatus represented above, and the other chamber is initially evacuated. The valve connecting the chambers is opened, and the gas expands at constant temperature to fill both chambers. Which of the following best describes AS for the process? A AS 0 because the gas particles become dispersed in a larger volume.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.141QP
Related questions
Question
100%
6
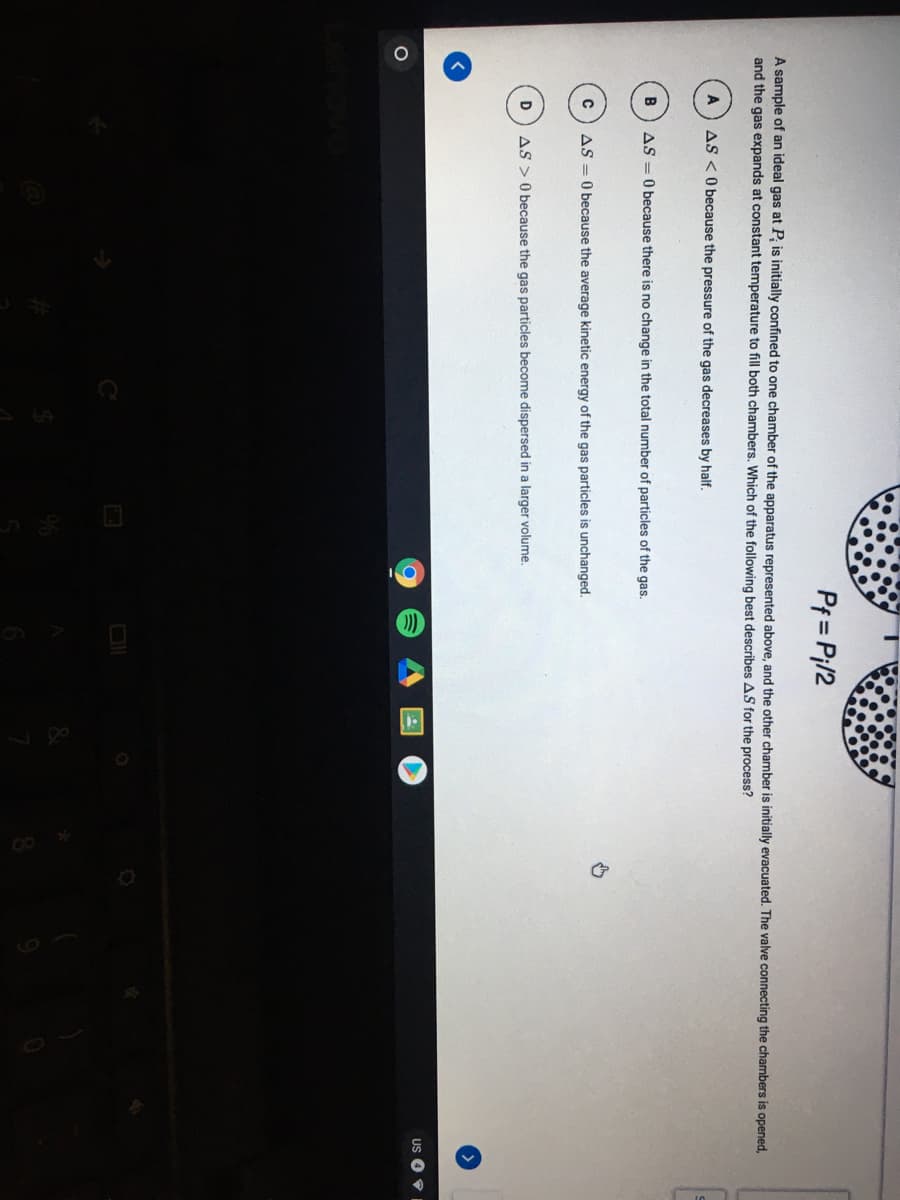
Transcribed Image Text:Pf = Pi/2
A sample of an ideal gas at P; is initially confined to one chamber of the apparatus represented above, and the other chamber is initially evacuated. The valve connecting the chambers is opened,
and the gas expands at constant temperature to fill both chambers. Which of the following best describes AS for the process?
A
AS <O because the pressure of the gas decreases by half.
AS = 0 because there is no change in the total number of particles of the gas.
AS = 0 because the average kinetic energy of the gas particles is unchanged.
AS > 0 because the gas particles become dispersed in a larger volume.
US O 9
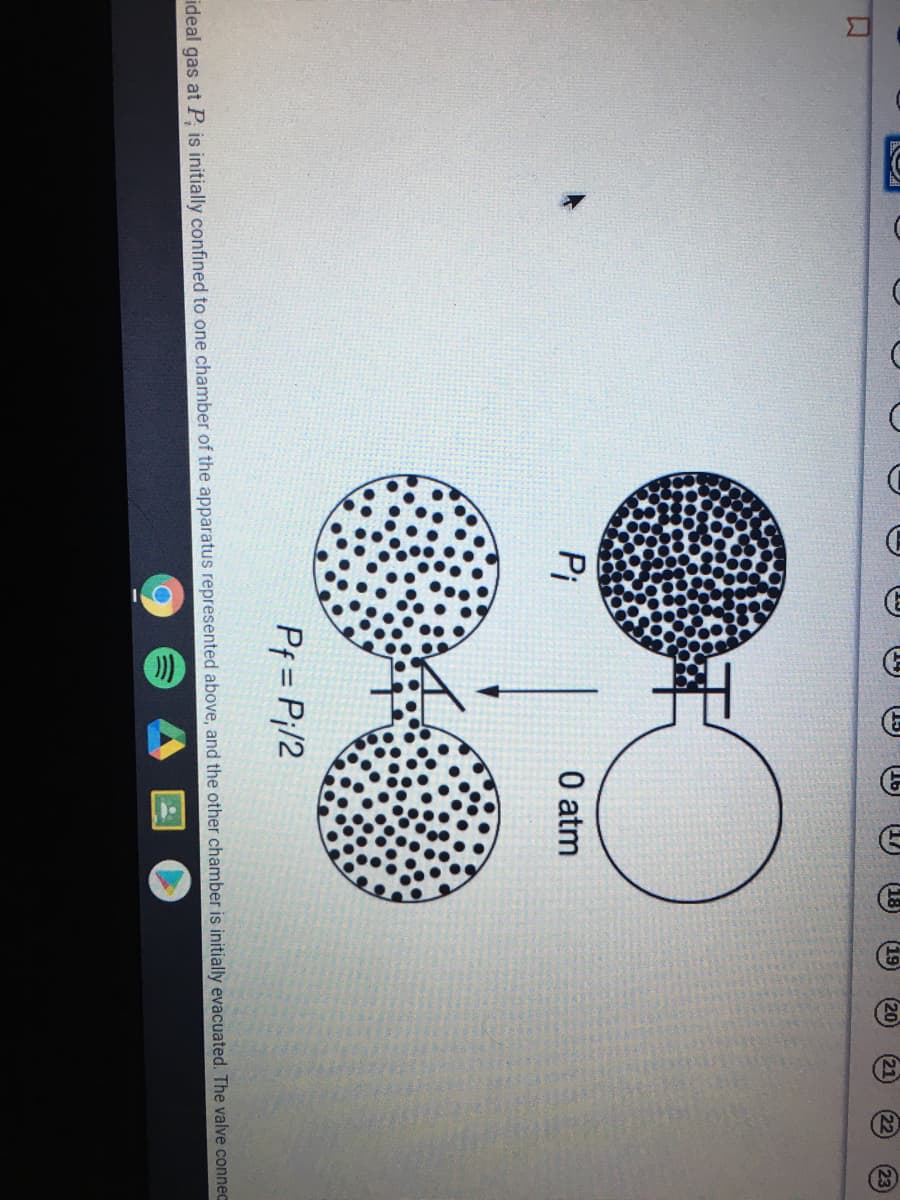
Transcribed Image Text:Pi
O atm
Pf = P¡/2
ideal gas at P, is initially confined to one chamber of the apparatus represented above, and the other chamber is initially evacuated. The valve connec
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER