ssolve 14.7 grams of sodium hydroxide pellets in 100 ml of carbon-dioxide free water. ool the solution to room temperature dd a freshly prepared saturated solution of barium hydroxide until no more precipitate forms. ake and filter. ansfer it in a 250 ml volumetric flask, then add sufficient quantity of carbon dioxide free water to ake 250 ml. dization eigh accurately about 5 grams of potassium biphthalate, previously crushed lightly and dried a 20 degrees Celsius for 2 hours and dissolve it in 75 ml of carbon dioxide free water. dd 2 drops of phenolphthalein TS. ansfer 50 ml of the sodium hydroxide solution in a Buret and fitrate the potassium biphthal lution with the sodium hydroxide solution until it produces a permanent pink color. ote: 24 ml NaOH solution was used to change the color from colorless to pink color e: Each ml of 1 N NaOH is equivalent to 204 mg of Potassium biphthalate Data Wt of KHP (g) MW of KHP meg of KHP e of sodium hydroxide solution used (ml) Trial 1 5.000 g 24.000
ssolve 14.7 grams of sodium hydroxide pellets in 100 ml of carbon-dioxide free water. ool the solution to room temperature dd a freshly prepared saturated solution of barium hydroxide until no more precipitate forms. ake and filter. ansfer it in a 250 ml volumetric flask, then add sufficient quantity of carbon dioxide free water to ake 250 ml. dization eigh accurately about 5 grams of potassium biphthalate, previously crushed lightly and dried a 20 degrees Celsius for 2 hours and dissolve it in 75 ml of carbon dioxide free water. dd 2 drops of phenolphthalein TS. ansfer 50 ml of the sodium hydroxide solution in a Buret and fitrate the potassium biphthal lution with the sodium hydroxide solution until it produces a permanent pink color. ote: 24 ml NaOH solution was used to change the color from colorless to pink color e: Each ml of 1 N NaOH is equivalent to 204 mg of Potassium biphthalate Data Wt of KHP (g) MW of KHP meg of KHP e of sodium hydroxide solution used (ml) Trial 1 5.000 g 24.000
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Based from the given problem, What is the Normality of the sodium hydroxide solution?
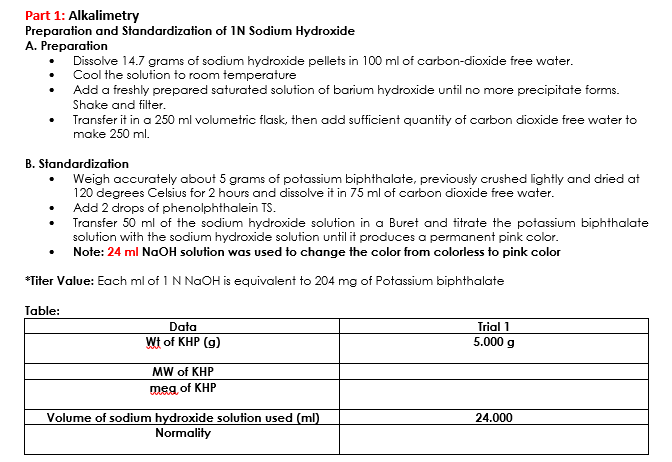
Transcribed Image Text:Part 1: Alkalimetry
Preparation and Standardization of 1N Sodium Hydroxide
A. Preparation
Dissolve 14.7 grams of sodium hydroxide pellets in 100 ml of carbon-dioxide free water.
Cool the solution to room temperature
Add a freshly prepared saturated solution of barium hydroxide until no more precipitate forms.
Shake and filter.
Transfer it in a 250 ml volumetric flask, then add sufficient quantity of carbon dioxide free water to
make 250 ml.
B. Standardization
Weigh accurately about 5 grams of potassium biphthalate, previously crushed lightly and dried at
120 degrees Celsius for 2 hours and dissolve it in 75 ml of carbon dioxide free water.
Add 2 drops of phenolphthalein TS.
Transfer 50 ml of the sodium hydroxide solution in a Buret and titrate the potassium biphthalate
solution with the sodium hydroxide solution until it produces a permanent pink color.
Note: 24 ml NaOH solution was used to change the color from colorless to pink color
*Titer Value: Each ml of 1 N NaOH is equivalent to 204 mg of Potassium biphthalate
Table:
Data
Wt of KHP (g)
MW of KHP
meg of KHP
Volume of sodium hydroxide solution used (ml)
Normality
Trial 1
5.000 g
24.000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY