Stainless steel is known and used for its ability to resist corrosion. More corrosion resistant stainless steels such as those used in building construction contain molybdenum as well as chromium. Chromium metal can be electroplated from an aqueous solution of potassium dichromate. A current of 6.00 A and a voltage of 4.5 V are used in the electroplating. The reduction half-reaction is: Cr₂07¹ (aq) + 14H+ + 12e → 2Cr(s) +7H₂O a. How many grams of chromium can be plated if the current is run for 48 minutes? b. How long will it take to completely convert 215 mL of 1.25 M K2Cr2O7 to elemental chromium?
Stainless steel is known and used for its ability to resist corrosion. More corrosion resistant stainless steels such as those used in building construction contain molybdenum as well as chromium. Chromium metal can be electroplated from an aqueous solution of potassium dichromate. A current of 6.00 A and a voltage of 4.5 V are used in the electroplating. The reduction half-reaction is: Cr₂07¹ (aq) + 14H+ + 12e → 2Cr(s) +7H₂O a. How many grams of chromium can be plated if the current is run for 48 minutes? b. How long will it take to completely convert 215 mL of 1.25 M K2Cr2O7 to elemental chromium?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 89QAP: An electrolysis experiment is performed to determine the value of the Faraday constant (number of...
Related questions
Question
Hi , i need your help to asnwer this
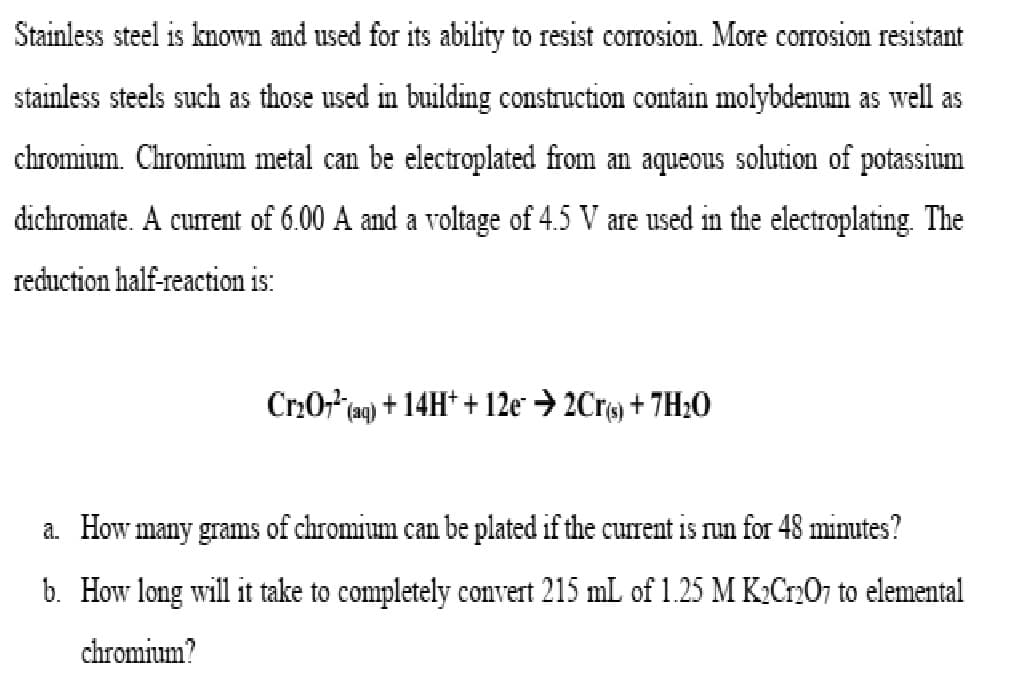
Transcribed Image Text:Stainless steel is known and used for its ability to resist corrosion. More corrosion resistant
stainless steels such as those used in building construction contain molybdenum as well as
chromium. Chromium metal can be electroplated from an aqueous solution of potassium
dichromate. A current of 6.00 A and a voltage of 4.5 V are used in the electroplating. The
reduction half-reaction
is:
Cr₂07¹ (aq) + 14H+ + 12e → 2Cr(s) + 7H₂0
a. How many grams of chromium can be plated if the current is run for 48 minutes?
b. How long will it take to completely convert 215 mL of 1.25 M K₂Cr2O7 to elemental
chromium?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning