Standard Cell Potential. Use tabulated data to calculate the standard cell potential, E° following redox reactions occurring in an electrochemical cell at 25°C. ALSO state whether or not the reaction is spontaneous as written. (The redox equations are alreadybalanced.) for the 3. a) Fe(s) Mn2(aq) Fe2 (aq)+ Mn(s) b) Mno (aq)4H (aq)Fe(s) MnO2(s) 2 H20(U) Fe (aq)
Standard Cell Potential. Use tabulated data to calculate the standard cell potential, E° following redox reactions occurring in an electrochemical cell at 25°C. ALSO state whether or not the reaction is spontaneous as written. (The redox equations are alreadybalanced.) for the 3. a) Fe(s) Mn2(aq) Fe2 (aq)+ Mn(s) b) Mno (aq)4H (aq)Fe(s) MnO2(s) 2 H20(U) Fe (aq)
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.46QE
Related questions
Question
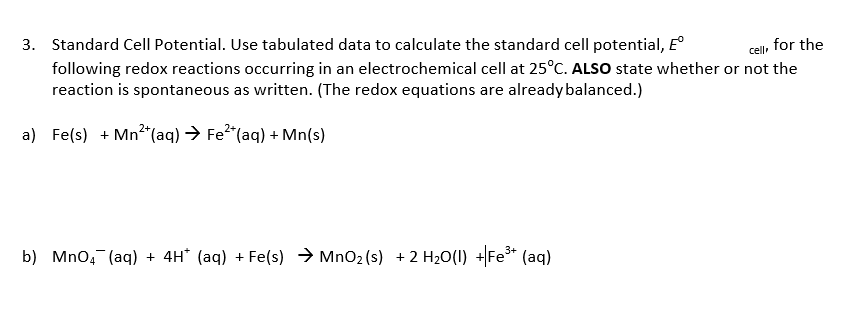
Transcribed Image Text:Standard Cell Potential. Use tabulated data to calculate the standard cell potential, E°
following redox reactions occurring in an electrochemical cell at 25°C. ALSO state whether or not the
reaction is spontaneous as written. (The redox equations are alreadybalanced.)
for the
3.
a) Fe(s) Mn2(aq) Fe2 (aq)+ Mn(s)
b) Mno (aq)4H (aq)Fe(s) MnO2(s) 2 H20(U) Fe (aq)
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 6 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning