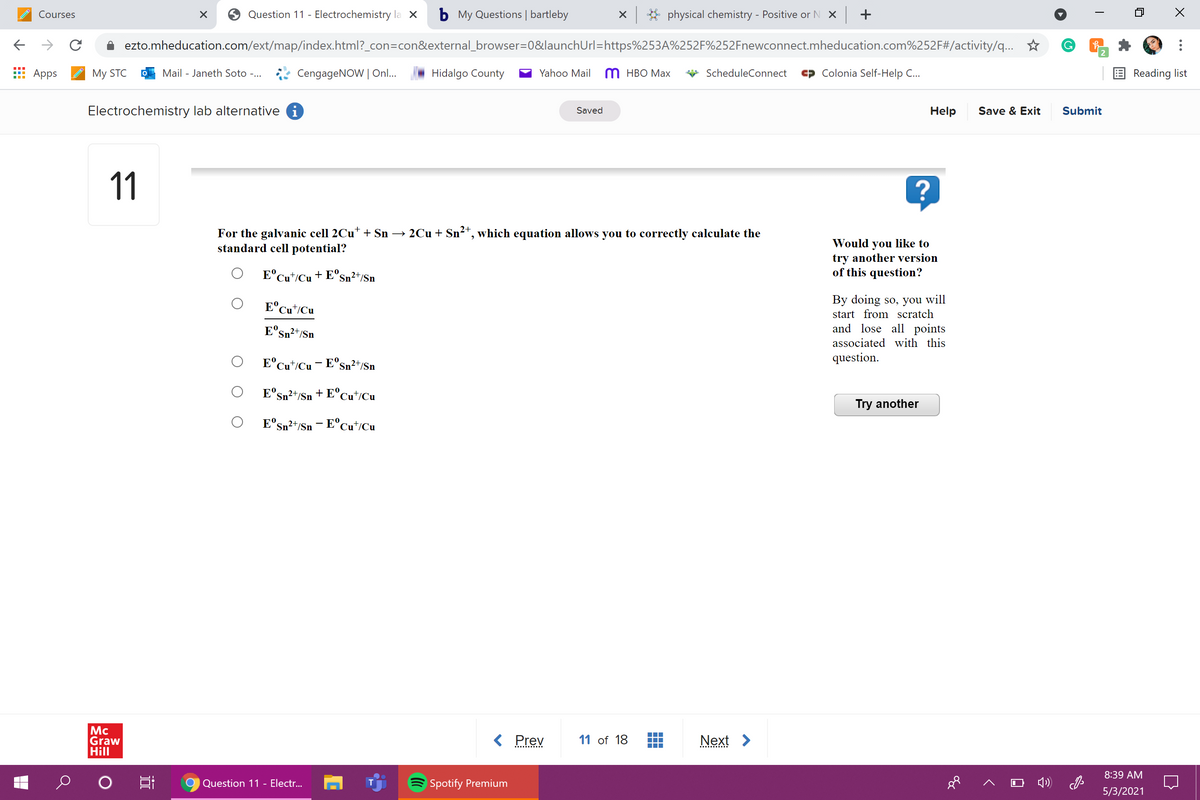
For the galvanic cell 2Cu* + Sn → 2Cu + Sn²“, which equation allows you to correctly calculate the standard cell potential? Would you like to try another version of this question? O E°cu/Cu+ E°sm²*/$n By doing so, you will start from scratch and lose all points associated with this question. E°Cu°ICu O E°Cu*/Cu- E°s»²*/Sn O E'sa?*Sn + E°cu*i©u Try another O E°Sn"/Sn - E°Cu*/Cu
For the galvanic cell 2Cu* + Sn → 2Cu + Sn²“, which equation allows you to correctly calculate the standard cell potential? Would you like to try another version of this question? O E°cu/Cu+ E°sm²*/$n By doing so, you will start from scratch and lose all points associated with this question. E°Cu°ICu O E°Cu*/Cu- E°s»²*/Sn O E'sa?*Sn + E°cu*i©u Try another O E°Sn"/Sn - E°Cu*/Cu
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.21QAP
Related questions
Question
Transcribed Image Text:Courses
Question 11 - Electrochemistry la X
b My Questions | bartleby
X * physical chemistry - Positive or N X +
ezto.mheducation.com/ext/map/index.html?_con=con&external_browser=0&launchUrl=https%253A%252F%252Fnewconnect.mheducation.com%252F#/activity/q...
2
Аpps
My STC
Mail - Janeth Soto -...
CengageNOW | On..
Hidalgo County
Yahoo Mail
M HBO Max
ScheduleConnect
Colonia Self-Help C..
E Reading list
Electrochemistry lab alternative i
Help
Save & Exit
Submit
Saved
11
For the galvanic cell 2Cu* + Sn → 2Cu + Sn²*, which equation allows you to correctly calculate the
standard cell potential?
Would you like to
try another version
of this question?
E°Cut/Cu + E°s
Sn²+/Sn
By doing so, you will
start from scratch
and lose all points
E°Cu*/Cu
E°sn²+/Sn
associated with this
E°Cu*/Cu- E°Sn²+/Sn
question.
E'sn²+/Sn
+ E°Cu*/Cu
Try another
E°Sn²t/Sn - E°Cu*/Cu
Mc
Graw
Hill
< Prev
11 of 18
Next >
8:39 AM
Question 11 - Electr...
Spotify Premium
5/3/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning