Standard reduction potentials for tin(II) and copper(Il) The standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard conditions. The more positive the reduction potential, the more easily the substance gains electrons. Consider the following: Sn2+ (aq) + 2e-Sn(s), Cu²+(aq) + 2e Cu(s), E' red -0.140 V E' red +0.337 V Part B What is the standard potential, E cell, for this galvanic cell? Use the given standard reduction potentials in your calculation as appropriate. Express your answer to three decimal places and include the appropriate units. > View Available Hint(s) Value Units E' cell
Standard reduction potentials for tin(II) and copper(Il) The standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard conditions. The more positive the reduction potential, the more easily the substance gains electrons. Consider the following: Sn2+ (aq) + 2e-Sn(s), Cu²+(aq) + 2e Cu(s), E' red -0.140 V E' red +0.337 V Part B What is the standard potential, E cell, for this galvanic cell? Use the given standard reduction potentials in your calculation as appropriate. Express your answer to three decimal places and include the appropriate units. > View Available Hint(s) Value Units E' cell
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter19: Electrochemistry
Section: Chapter Questions
Problem 19.140QP
Related questions
Question
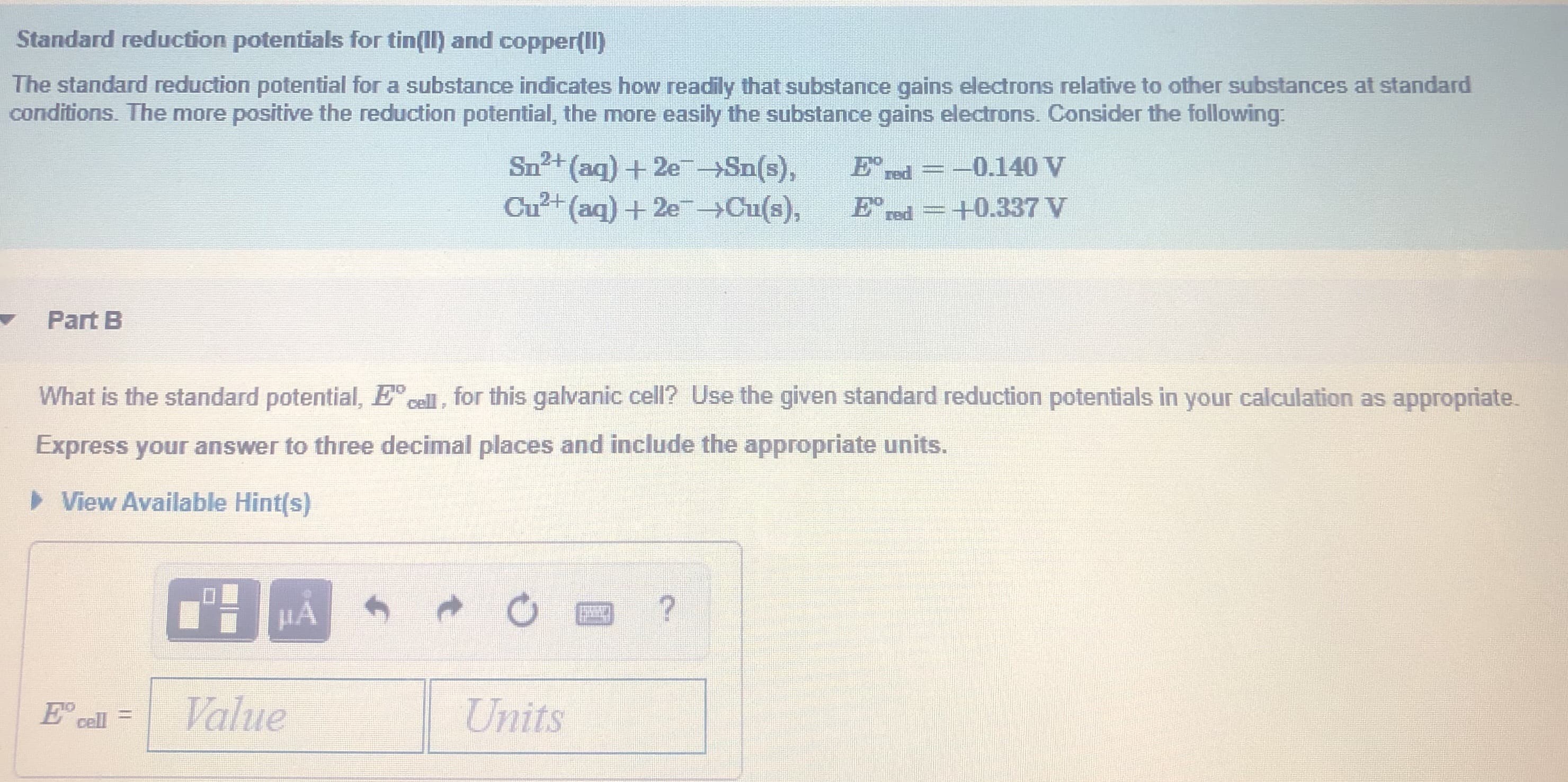
Transcribed Image Text:Standard reduction potentials for tin(II) and copper(Il)
The standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard
conditions. The more positive the reduction potential, the more easily the substance gains electrons. Consider the following:
Sn2+ (aq) + 2e-Sn(s),
Cu²+(aq) + 2e Cu(s),
E' red
-0.140 V
E' red
+0.337 V
Part B
What is the standard potential, E cell, for this galvanic cell? Use the given standard reduction potentials in your calculation as appropriate.
Express your answer to three decimal places and include the appropriate units.
> View Available Hint(s)
Value
Units
E' cell
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning