Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.10QAP
Related questions
Question

Transcribed Image Text:2.
Determine the number of moles of Phosphate in your water sample.
3.
Determine the ppm of Phosphorus in your original water sample.
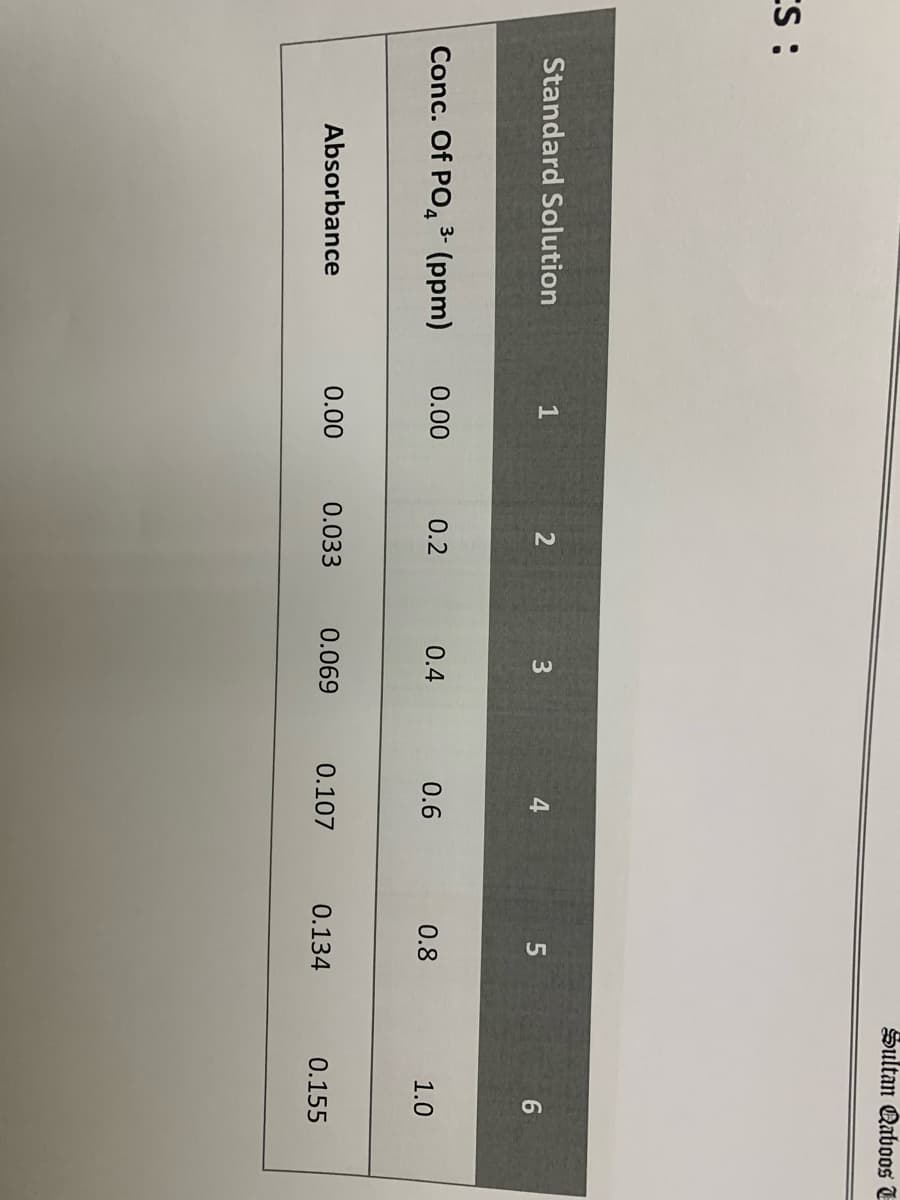
Transcribed Image Text:Sultan Qaboos E
ES :
Standard Solution
3
Conc. Of PO, 3- (ppm)
0.00
0.2
0.4
0.6
0.8
1.0
Absorbance
0.00
0.033
0.069
0.107
0.134
0.155
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

