Stannous fluoride, or Tn(II) fluoride, is a compound commonly used in toothpastes with similar in function and activity to sodium fluoride (NaF), the conventionally added ingredient in toothpastes, but has been shown to be more effective. Draw the predicted 1Sn and F NMR spectra of stannous fluoride. The table shows the atural abundance of the NMR active nuclei of interest with their magnetogyric ratios. Magnetogyric tatio (10 rac/1s) NMR active NA Isotope 1155n 0.35 1/2 -8.80 1175n 7.61 1/2 -9.59 1195n 8.58 1/2 -10.01 19F 100 1/2 25.18 Stannous Fluoride (SnF2)
Stannous fluoride, or Tn(II) fluoride, is a compound commonly used in toothpastes with similar in function and activity to sodium fluoride (NaF), the conventionally added ingredient in toothpastes, but has been shown to be more effective. Draw the predicted 1Sn and F NMR spectra of stannous fluoride. The table shows the atural abundance of the NMR active nuclei of interest with their magnetogyric ratios. Magnetogyric tatio (10 rac/1s) NMR active NA Isotope 1155n 0.35 1/2 -8.80 1175n 7.61 1/2 -9.59 1195n 8.58 1/2 -10.01 19F 100 1/2 25.18 Stannous Fluoride (SnF2)
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL3: Carbon (13c) Nmr Spectroscopy
Section: Chapter Questions
Problem 12CTQ
Related questions
Question
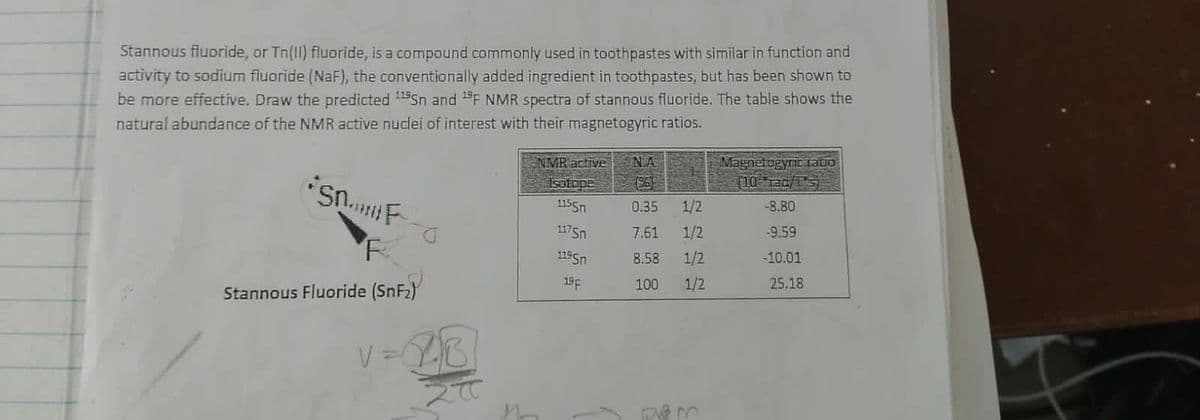
Transcribed Image Text:Stannous fluoride, or Tn(1l) fluoride, is a compound commonly used in toothpastes with similar in function and
activity to sodium fluoride (NaF), the conventionally added ingredient in toothpastes, but has been shown to
be more effective. Draw the predicted 11Sn and 1F NMR spectra of stannous fluoride. The table shows the
natural abundance of the NMR active nuclei of interest with their magnetogyric ratios.
NIMR active
Magnetogyric tatio
(10 rad/s)
NA
E Isotope
1155n
0.35
1/2
-8.80
117Sn
7.61
1/2
-9,59
1195n
8.58
1/2
-10.01
19F
100
1/2
25.18
Stannous Fluoride (SnF2)
V =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning