sted in the outline of the Periodic Table below: mat can you say about these elements without knowing exactly which they are? Use that knowledge to answer the questions in the following table, if possible. portant: do not try to figure out exactly which elements are marked, and then use your knowledge of the properties of each specific element. You don't need You will also be marked wrong for any answer, correct or not, that can't be determined from the rough location of each marked element in the Periodic Table, O Element X Element Y Which element in the gas phase is more likely to glow green or yellow in in flame? ? O Can't say without more information. O Element X Which element is more likely to form an ionic compound with chlorine? O Element Y O Can't say without mere information. O Element X Which element in the solid state is probably brittle, so that it breaks before bending? O Element Y O Can't say without more information. planation Check
sted in the outline of the Periodic Table below: mat can you say about these elements without knowing exactly which they are? Use that knowledge to answer the questions in the following table, if possible. portant: do not try to figure out exactly which elements are marked, and then use your knowledge of the properties of each specific element. You don't need You will also be marked wrong for any answer, correct or not, that can't be determined from the rough location of each marked element in the Periodic Table, O Element X Element Y Which element in the gas phase is more likely to glow green or yellow in in flame? ? O Can't say without more information. O Element X Which element is more likely to form an ionic compound with chlorine? O Element Y O Can't say without mere information. O Element X Which element in the solid state is probably brittle, so that it breaks before bending? O Element Y O Can't say without more information. planation Check
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 128QRT
Related questions
Question
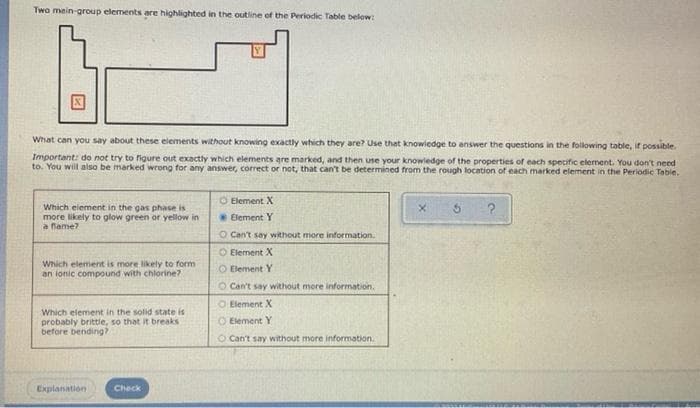
Transcribed Image Text:Two main-group elements are highlighted in the outline of the Periodic Table below:
What can you say about these elements without knowing exactly which they are? Use that knowledge to answer the questions in the following table, if possible.
Important: do not try to figure out exactly which elements are marked, and then use your knowledge of the properties of each specific element. You don't need
to. You will also be marked wrong for any answer, correct or not, that can't be determined from the rough location of each marked element in the Periodic Table.
Element X
Element Y
Which element in the gas phase is
more likely to glow green or yellow in
a flame?
X 5
O Can't say without more information.
O Element X
Which element is more likely to form
an ionic compound with chlorine?
O Element Y
O Can't say without more information.
O Element X
Which element in the solid state is
probably brittle, so that it breaks
before bending?
Element Y
Can't say without more information.
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning