Q: What is the half-life of a first-order reaction with a rate constant of 7.40×10−4 s−1?
A: Given that, Rate constant for a first order reaction K = 7.40×10-4 s-1 half life time period for…
Q: 4. Given that the first order rate constant for the overall decomposition of ethyl bromide is k =…
A: Introduction : First order reaction can be defined as one whose rate is directly proportional to…
Q: The rate law for the following reaction has been experimentally determined to be third order: 2NO(g)…
A:
Q: What is the rate constant of a first-order reaction when 20.0% of a reactant remains after 38.5 s?
A:
Q: After 55.0 min, 11.0% of a compound has decomposed. What is the half‑life of this reaction assuming…
A:
Q: he kinetics of the reaction, A + B → P, were studied and it was determined that the reaction rate…
A: Rate law gives the relation between rate of the reaction and concentration of the reactants.
Q: If the rate law for the reaction 2A + 3B → C, is rate = k, then the reaction is ___ order in terms…
A: The rate of reaction depends on the concentration of the reactant. Sometimes it is also independent…
Q: What is the rate law for the following mechanism in terms of the overall rate constant k? A +B Step…
A: Answer: According to the law of mass action, rate of reaction for any step will directly be…
Q: the rate law of decomposing H2O2 is: rate = k[H2O2]3[KI]3 How much the did concentration multiply?
A: If the concentration is doubled the rate becomes 64 times as the order is 3 w.r.t both H2O2 and KI
Q: At body temperature (37°C), the rate constant of an enzymecatalyzed decomposition is 2.3 x 1014…
A: Given data: Body temperature = 370C = 310.15K Rate constant for enzyme catalyzed =2.3 x 1014 Gas…
Q: Given: A + 3B 2C +D This reaction is first order with respect to reactant A and second order with…
A: Given, Chemical Reaction : A + 3B --> 2C + D First order with respect to [A] & First order…
Q: A certain second-order reaction (B→products) has a rate constant of 1.80x10-3 M s1 at 27°C and an…
A:
Q: Consider a reaction A + B → C with corresponding rate law rate = k[A]n [B]m If n = 2, m = 1: (a)…
A: Given : Rate law is given as : rate = k[A]n [B]m
Q: In a study of nitrosyl halides, a chemist proposes the fol-lowing mechanism for the synthesis of…
A: The rate law relates the rate constant and concentration of reactants and products taking part in…
Q: 3. The rate constant for the first-order decomposition of cyclobutane, C.H, at 500 °C is 9.2 x 10°s…
A: "Since there are multiple subparts, we will answer only the first three subparts. If you require…
Q: 3. The decomposition of a herbicide A in a water at 20° C is a 1st-order reaction with half-life of…
A: Given:Half-life = 2 months.Initial concentration No = 1.6×10-6 MFinal concentration N = 1×10-7 M
Q: In a study of the enzyme-catalysed oxidation of ethanol, the molar concentration of ethanol…
A: Given The reaction is a first order reaction Initial concentration = 220 mmol/dm3 Final…
Q: The thermal decomposition of nitryl chloride, NO2CI, is believed to occur by the following…
A: The decomposition of nitryl chloride follows the mechanism as follows: (i) NO2Cl(g)→k1 NO2…
Q: Which of the following graphs of the rate constant (k) and the temperature would give a straight…
A: We will use arrhenious Equation for this plot of graph .
Q: A kinetics study of a reaction, A +B -C, is found to be zeroth order with respect to A and second…
A: Rate of the reaction depends on order of the reaction
Q: 1) The rate constant for the first-order decomposition of a compound A in the reaction A→P is k =…
A: Given Order of reaction = 1 Rate Constant (K) = 2.78 × 10-7 sec-1 Half life ( t1/2) = ?
Q: 5. What is the value for the rate constant, k? k = k'/1* = %3D %3D k %3D San = k[OH']*[ C2H3,N,J"…
A: 5) k = k' / [1]x substituting the values of K' and x we have k = 0.1583 / [1]1 = 0.1583 M-1 s-1 k =…
Q: 4. 5. given. Based on this information, write the rate law for the overall reaction: Hb(H+)4 (aq) +4…
A: According to guideline only one question answer if any doubt these question you can ask me
Q: A certain first-order reaction (A→products) has a rate constant of 7.50×10−3 s^−1 at 45∘C. How many…
A: Given: Order of reaction = 1 Rate constant of the reaction = 7.50 X 10-3 s-1. And final…
Q: A particular first-order reaction has a rate constant of 1.35 x 102 s1 at 25.0 °C. What is the…
A:
Q: Given the following reactions and the corresponding rate laws, in which of the reactions might the…
A: In the second reaction (b) the coefficient of PCl3 and Cl2 is one and the rate law involves both…
Q: How long would it take for the reactant of a first-order reaction todecrease to one-half its initial…
A: This problem can be solved by using the rate law for the first order reaction which is given as…
Q: Americium-241 is used in smoke detectors. It has a first-order rate constant for radioactive decay…
A: Since the reaction is first order, then the relationship between rate constant and half-life period…
Q: Explain the following terms:(i) Rate constant (k)(it) Half life period of a reaction (t1/2)
A: (1) Rate Constant (K): It is a proportionality constant which deals with the relationship between…
Q: 2A + B + C à D If the rate law for this reaction is r = k[A]2[B]1, what is the order of reaction…
A: The reaction given is, => 2 A + B + C → D Given: Rate law of the reaction is, => Rate =…
Q: 7. The following 3-step mechanism has been proposed for the reaction of chlorine and chloroform:…
A:
Q: The hydrolysis of the sugar sucrose to the sugars glucose and fructose follows a first-order rate…
A: The convergences of glucose, fructose, and sucrose after a 0.150 M watery arrangement of sucrose…
Q: rate laws for certain enzyme-activated reactions in your body have a specific rate constant k, with…
A: Given -> Rate constant= K Unit of Rate constant (K) = M/s
Q: Hydrogen and nitrogen(1I) oxide react according to the following equation. 2H,(g) + 2NO(g) = N,(g) +…
A: for the given reaction, 2H2(g) + 2NO(g) ⇔ N2(g) + 2H2O(g) Rate of the reaction, Rate = k…
Q: The decomposition of nitrogen dioxide, 2 NO2(g) 2 NO(g) + O2(g) is second order with a rate constant…
A: Second order rate constant K= 1/t . x /(a-x) Half life t1/2 = 1/a K
Q: The isomerization of methyisocyanide, CH3NC, to acetonitrile, CH;CN, is a first-order reaction. If…
A: Nm
Q: 4. For the acid hydrolysis of ethyl acetate, the rate constant at different temperatures is given in…
A: The data given is,
Q: The decomposition of O3 is a second order reaction with a rate constant of 50.5 M hr-1.…
A: The given data contains, Rate constant = 50.5 M hr-1 Concentration = 5.609×10-6M.
Q: It is very desirable that pesticides eventually decompose in the environment to give harmless…
A: The half-life of a reactant species is the time required to consume half of its initial…
Q: Sulfuryl chloride undergoes first order decomposition at 320.C with a half life of 8.75 hours.❏…
A: First order reactions are those in which rates are directly depend on the concentration of reactant.
Q: The isomerization reaction of cyclopropane to propene at 500°C follows first-order kinetics with a…
A: The rate constant equation for the first-order reaction can be given as follows: k=2.303tlogaa-x…
Q: Given the following proposed mechanism, predict the rate law for the overall reaction. A2 + 2 B →…
A: Rate laws are the mathematical expressions that help to describe the relationship of the reactant…
Q: 4. A reaction is first order in A. If the rate constant of the reaction is 6.00 x 10s', what is the…
A: Order of reaction = 1 st order Rate constant (k) = 6.00 × 10-3 s-1 Half Life (t1/2) = ?
Q: A certain second-order reaction (B→products) has a rate constant of 2.00×10−3 M^−1⋅s^−1 at 27∘C and…
A:
Q: If the rate laws are expressed with (i) concentrations in moles per cubic decimetre, (ii) pressures…
A: If the rate laws are expressed with (i) concentrations in moles per cubic decimeter : Unit of a…
Q: A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.264…
A:
Q: (Q83) The conversion of ammonium cyanate into urea is a second order reaction. If the initial…
A: Initial contact of ammonium cyanate = 1.796M Concentration of ammonium cyanate after 38 minutes =…
Q: 5. What is the value for the rate constant, k? k = k'/1* = k = k"/0.5* = kavg r = k[OH]*[…
A: 5) k = k' / [1]^x substituting the values of K' and x we have k = 0.1583 / [1]^1 = 0.1583 M^-1s^-1 k…
Q: For the reaction, B ==> products (a) What is the rate expression for the above chemical reaction…
A: The reaction taking place is given as, => B -------> Products
What is the rate law for the following mechanism in terms of the overall rate constant, K?
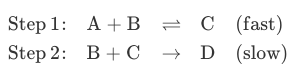
Step by step
Solved in 2 steps with 2 images

- Reaction rate for the chemical reaction 2A + B → A2B Select one: a. V = KCA .CB b. V = KCB c. V = KCA2 d. V = KCA2 .CBChemical reaction rates generally double for a 10-K increase in temperature. How large an increase is that in Fahrenheit? what is the answer? a. 10 deg F b. 5.56 K c. 5.56 deg R d. 18 deg FPls show eork, will rate, D,E,F
- rate law is r=k[ClO2][OH-]rate= k[A]x determine the value of x if the rate doubles when [A] is doubled. determine the value of x if no change in rate occurs when [A] is doubledWhich of the reaction mechanisms is consistent with the energy diagram? A. 2A−→−fast BB−→−fast CC−→−−slow D B. 2A−→−fast BB−→−−slow C C. A+B⟶C D. A+B−→−−slow CC−→−fast D
- Rate = k[acetone]1[I2]0[H+]1 rate = 0.001M/ 332.26s = 3.01 x 10-6 my question is what is the S.I unit of this answer? 3.01 x 10-6 = k[0.80M]1 [0.001M]0 [0.20]1 k= (3.01 x 10-6)/ (0.16) k = 1.88 x 10-5 also S.I unit of this answer with clear explanation and stepsConsider the following mechanism: Cl2 → Cl+ + Cl- Cl- + H2S → HCl + HS- Cl+ + HS- → HCl + S What is the role of Cl+? A. product B. intermediate C. catalyst D. reactantA reaction proceeds by the following mechanism: NO2Cl+Cl→NO2Cl2 NO2Cl2→NO2+Cl2 What is the overall reaction? a. NO2Cl+Cl→NO2+Cl2 b. NO2Cl+Cl+NO2Cl2→NO2+Cl2+NO2Cl2 c. NO2Cl+Cl+2NO2Cl2→NO2+Cl2 d. NO2Cl+Cl+NO2+Cl2→2NO2Cl2



