Student Name: Experimental: Part I: (standardization of NaOH) Mass of KHP: Moles of KHP: Initial Reading of buret: (NaOH) Final Reading of buret: Volume of NaOH used: Moles of NaOH: Molarity of NaOH: Average Molarity of NaOH: CAL Trial 1 Titration of Vinegar 0.263g Trial 2 10.00 mL 0.283 g 23.30ML 23.30ML 36.70mL 13.30m² 13.40mb OLD Trial 3 (if needed) (and if time allows)
Student Name: Experimental: Part I: (standardization of NaOH) Mass of KHP: Moles of KHP: Initial Reading of buret: (NaOH) Final Reading of buret: Volume of NaOH used: Moles of NaOH: Molarity of NaOH: Average Molarity of NaOH: CAL Trial 1 Titration of Vinegar 0.263g Trial 2 10.00 mL 0.283 g 23.30ML 23.30ML 36.70mL 13.30m² 13.40mb OLD Trial 3 (if needed) (and if time allows)
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 31E
Related questions
Question
100%
Titration of Vinegar resubmit, added NaOH used

Transcribed Image Text:pers
to
Part II: (Titration of Vinegar)
Volume of Vinegar:
Initial Reading of buret:
(NaOH)
Final Reading of buret:
Volume of NaOH used:
Moles of acetic acid in sample:
Grams of acetic acid in sample:
Weight to Volume Percentage:
Trial 1
Titration of Vinegar
2.00mL
5.20ml
19.30mL
3.20mL
mass of acetic acid in grams X 100%
volume of vinegar in ml
Calculations: (moles of acetic acid in sample, grams of acetic acid in sample, weight to volume percentage of acetic acid
in vinegar sample)
14.328% - your w/V%. |
4.328%
Percentage error in your determination of the weight to volume percentage of acetic acid in vinegar:
Your lab instructor will have the true weight to volume percentage.
True w/V%. = 4.328%
% error =
When you titrated the vinegar, the instructions said to "add about 25 mL of water". Which of the following best explains
the result if you had added 30 mL of water?
a) The weight volume percent would be higher than actual because the greater volume titrated would contain more
vinegar.
b) The weight volume percent would be lower than actual because the greater volume would dilute the vinegar, so
less titrant would be needed.
c) The weight volume percentage would be lower than actual because the greater volume would dilute the added
sodium hydroxide
d) The weight volume percentage would be unaffected because the added water did not contain the vinegar- the 2.00
mL of vinegar was added in a separate step with a precise delivery device.
s lai
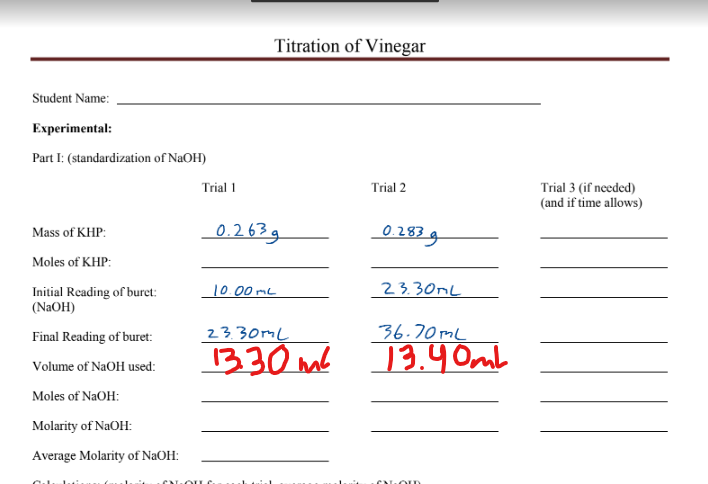
Transcribed Image Text:Student Name:
Experimental:
Part I: (standardization of NaOH)
Mass of KHP:
Moles of KHP:
Initial Reading of buret:
(NaOH)
Final Reading of buret:
Volume of NaOH used:
Moles of NaOH:
Molarity of NaOH:
Average Molarity of NaOH:
CAL
Trial 1
Titration of Vinegar
0.263g
Trial 2
10.00 mL
0.283 g
23.30ML
23.30ML
36.70mL
13.30m² 13.40mb
OLD
Trial 3 (if needed)
(and if time allows)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning