Sub O Previous Page 9 of 10 Next. Consider the following reaction in acidic solution, along with the half-reactions: I(ag) + ClO (ag) I,(ag) + CI (ag) Half-reactions: CiO + CI After determining the half-reactions, the next step in balancing oxidation-reduction equations using the half-reaction method is to balance each half-reaction. Select the correctly balanced half-reactions.for the reaction above: (Select all that apply) O CIO (ag) + CI (aq) + H20(1) O CIO (ag) 2H (aq) + CIO (aq) + CI (ag) + H20(1) O 2e + 2H* (ag) + CIO (ag) CI (ag) + H20(1) O 31 (ag) →5(ag) + 2e O 31 (aq) +5(aq) CI (ag) + H20(1) + 2H* (ag) https://ais-asp.cengage.info/als-asp/take Save and Exit
Sub O Previous Page 9 of 10 Next. Consider the following reaction in acidic solution, along with the half-reactions: I(ag) + ClO (ag) I,(ag) + CI (ag) Half-reactions: CiO + CI After determining the half-reactions, the next step in balancing oxidation-reduction equations using the half-reaction method is to balance each half-reaction. Select the correctly balanced half-reactions.for the reaction above: (Select all that apply) O CIO (ag) + CI (aq) + H20(1) O CIO (ag) 2H (aq) + CIO (aq) + CI (ag) + H20(1) O 2e + 2H* (ag) + CIO (ag) CI (ag) + H20(1) O 31 (ag) →5(ag) + 2e O 31 (aq) +5(aq) CI (ag) + H20(1) + 2H* (ag) https://ais-asp.cengage.info/als-asp/take Save and Exit
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 101QAP: Use Table 17.1 to answer the following questions. Use LT (for is less than), GT (for is greater...
Related questions
Question
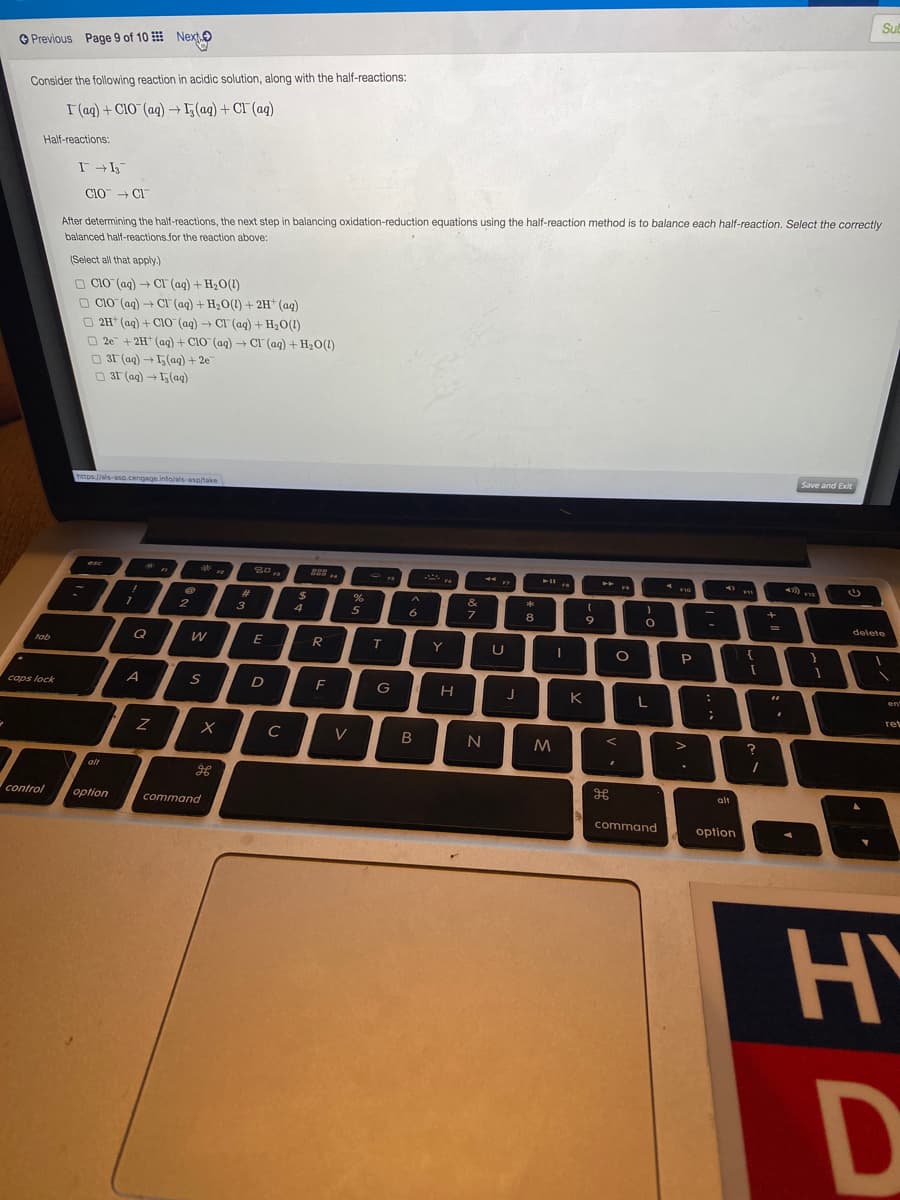
Transcribed Image Text:Sub
O Previous Page 9 of 10 Next.
Consider the following reaction in acidic solution, along with the half-reactions:
I(ag) + ClO (ag) I,(ag) + CI (ag)
Half-reactions:
CiO + CI
After determining the half-reactions, the next step in balancing oxidation-reduction equations using the half-reaction method is to balance each half-reaction. Select the correctly
balanced half-reactions.for the reaction above:
(Select all that apply)
O CIO (ag) + CI (aq) + H20(1)
O CIO (ag)
2H (aq) + CIO (aq) + CI (ag) + H20(1)
O 2e + 2H* (ag) + CIO (ag) CI (ag) + H20(1)
O 31 (ag) →5(ag) + 2e
O 31 (aq) +5(aq)
CI (ag) + H20(1) + 2H* (ag)
https://ais-asp.cengage.info/als-asp/take
Save and Exit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax