4) The picture is from the lecture slides. It is a few of the standard half-cell reactions from Table 13-7 in your book (and lecture slide), and includes the extreme E°s, and some middle ones. Table of Standard Reduction Potential s Hall-reaction E (V) 10, + 2H* + 2e H,0 P Fe + e- Fe?* NO + 2H* + 2e NO; + H,0 Cytochrome f (Fe") +e cytochrome f(Fe) 0.816 0.771 0.421 0.365 0.031 Fumarate + 2H + 2e succinate 2H + 2e- H, Cat standard conditions, pH 0) Crotonyl-CoA + 2H" + 2e butyryl-CoA 0.000 -0.015 a-Ketoglutarate + CO, + 2H* + 2e isccitrate 2H* + 20 Hạ (at pH 7) Ferredoxin (Fe) +e ferredoxin (Fe) -0.38 -0.414 -0.432 5) Which reduction is the most spontaneous; meaning which reduction is associated with the most negative AGo. 6) Pick the two reactions that when combined as a balanced redox reaction will produce the most free energy. Write that balanced reaction (Hint, run ferredoxin (Fe2*) backwards and use this as your source of electrons) IOTTOM MIDDLE TOP
4) The picture is from the lecture slides. It is a few of the standard half-cell reactions from Table 13-7 in your book (and lecture slide), and includes the extreme E°s, and some middle ones. Table of Standard Reduction Potential s Hall-reaction E (V) 10, + 2H* + 2e H,0 P Fe + e- Fe?* NO + 2H* + 2e NO; + H,0 Cytochrome f (Fe") +e cytochrome f(Fe) 0.816 0.771 0.421 0.365 0.031 Fumarate + 2H + 2e succinate 2H + 2e- H, Cat standard conditions, pH 0) Crotonyl-CoA + 2H" + 2e butyryl-CoA 0.000 -0.015 a-Ketoglutarate + CO, + 2H* + 2e isccitrate 2H* + 20 Hạ (at pH 7) Ferredoxin (Fe) +e ferredoxin (Fe) -0.38 -0.414 -0.432 5) Which reduction is the most spontaneous; meaning which reduction is associated with the most negative AGo. 6) Pick the two reactions that when combined as a balanced redox reaction will produce the most free energy. Write that balanced reaction (Hint, run ferredoxin (Fe2*) backwards and use this as your source of electrons) IOTTOM MIDDLE TOP
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter32: Voltaic Cell Measurements
Section: Chapter Questions
Problem 2ASA
Related questions
Question
100%
Question in photo
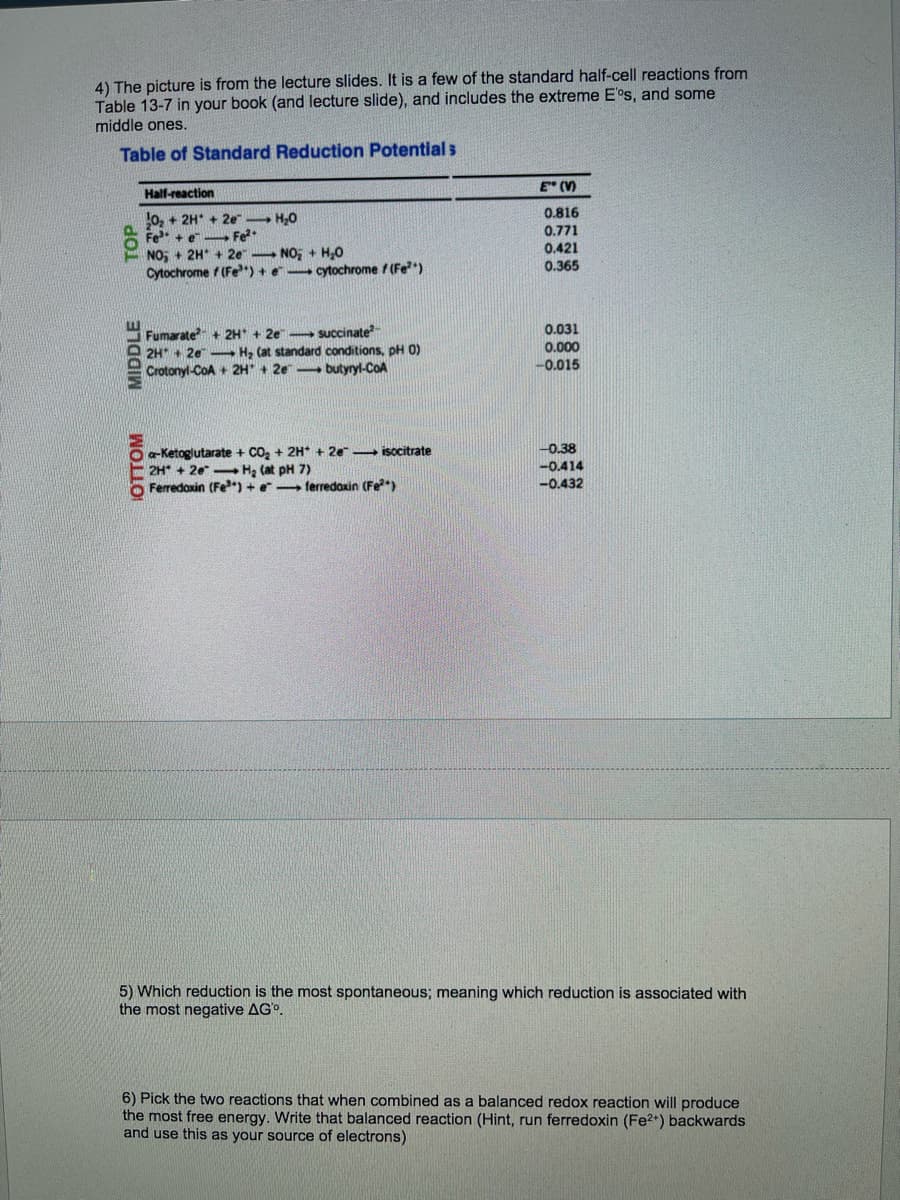
Transcribed Image Text:4) The picture is from the lecture slides. It is a few of the standard half-cell reactions from
Table 13-7 in your book (and lecture slide), and includes the extreme E°s, and some
middle ones.
Table of Standard Reduction Potential s
Half-reaction
E" (V)
0.816
0, + 2H* + 2e H0
Fe + e Fe
NO, + 2H* + 2e NO, + H,0
Cytochrome f (Fe") + e cytochrome f (Fe)
0.771
0.421
0.365
Fumarate + 2H + 2e succinate
2H + 2e- H, (at standard conditions, pH 0)
Crotonyl-CoA + 2H* + 2e - butyryl-CoA
0.031
0.000
-0.015
0.38
a-Ketoglutarate + CO, + 2H* + 2e isocitrate
2H* + 20- H, (at pH 7)
Ferredoxin (Fe) + e ferredaxin (Fe)
-0.414
-0.432
5) Which reduction is the most spontaneous; meaning which reduction is associated with
the most negative AG°.
6) Pick the two reactions that when combined as a balanced redox reaction will produce
the most free energy. Write that balanced reaction (Hint, run ferredoxin (Fe2+) backwards
and use this as your source of electrons)
IOTTOM
MIDDLE
TOP
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning