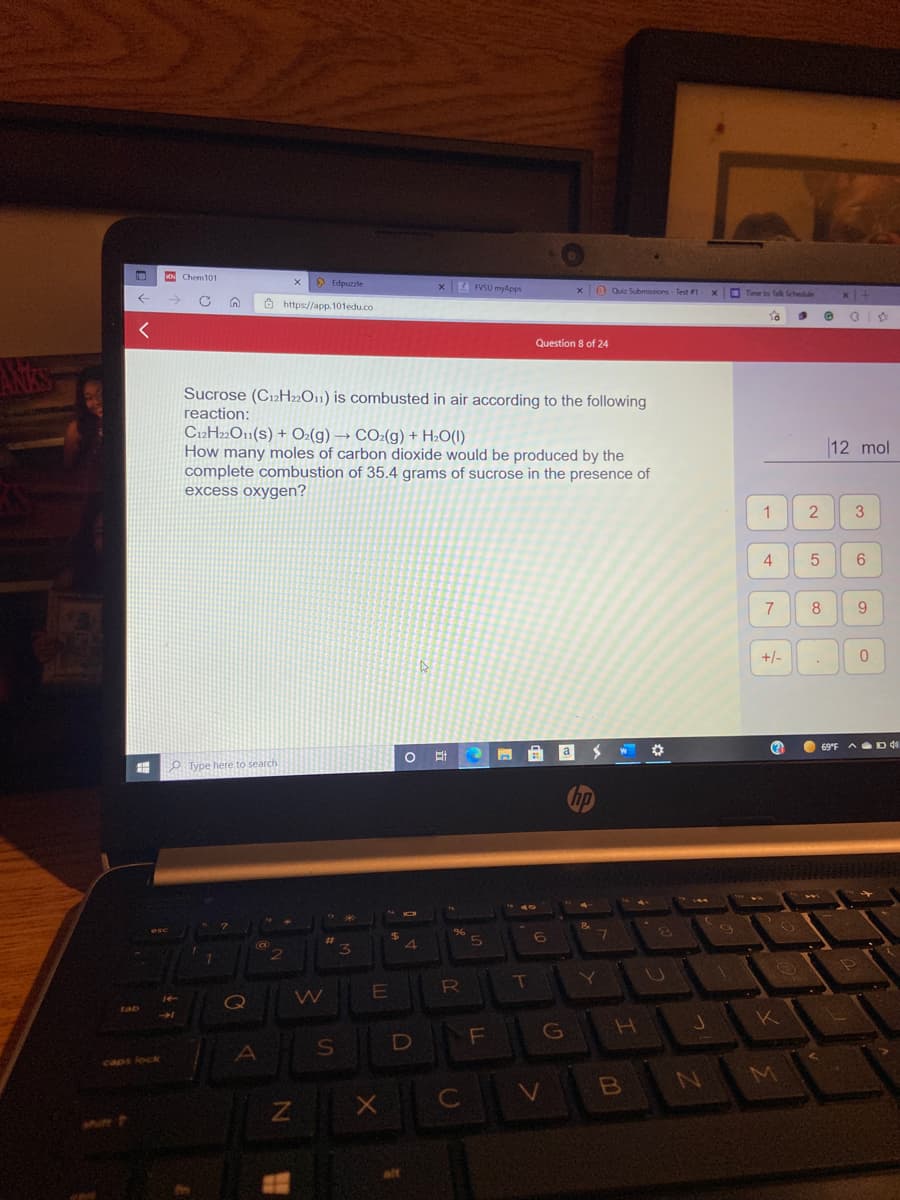
Sucrose (C12H»O11) is combusted in air according to the following reaction: C2H»O1(s) + O:(g) → CO:(g) + H:O(I) How many moles of carbon dioxide would be produced by the complete combustion of 35.4 grams of sucrose in the presence of excess oxygen?
Sucrose (C12H»O11) is combusted in air according to the following reaction: C2H»O1(s) + O:(g) → CO:(g) + H:O(I) How many moles of carbon dioxide would be produced by the complete combustion of 35.4 grams of sucrose in the presence of excess oxygen?
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter21: Rates Of Chemical Reactions, Ii. A Clock Reaction
Section: Chapter Questions
Problem 2ASA
Related questions
Question
Transcribed Image Text:K Chem101
> Edpuzzle
xE FVSU myApps
x0 Quiz Submissions - Test 1
x Time to f Schedule
A https://app. 101edu.co
Question 8 of 24
ANkS
Sucrose (C12H»O11) is combusted in air according to the following
reaction:
C12H2»O11(s) + O:(g) → CO2(g) + H2O(I)
How many moles of carbon dioxide would be produced by the
complete combustion of 35.4 grams of sucrose in the presence of
excess oxygen?
12 mol
1
2
3
4
6
7
8
+/-
O 69°F A D d
a
O Type here to search
hp
(0
Y
RO
Q
W
G
alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning