Suka-tuba is a vinegar variety derived by fermenting the sap of a coconut tree. You are tasked to analyze its acetic acid Describe the preparation of 5.0 M NaOH stock solution from analytical grade NaOH pellets. Describe the preparation of 0.10 M NaOH solution from the stock solution. How much KHP was used per trial. (purity KHP: 99.0%).
Suka-tuba is a vinegar variety derived by fermenting the sap of a coconut tree. You are tasked to analyze its acetic acid Describe the preparation of 5.0 M NaOH stock solution from analytical grade NaOH pellets. Describe the preparation of 0.10 M NaOH solution from the stock solution. How much KHP was used per trial. (purity KHP: 99.0%).
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.47QAP
Related questions
Question
Suka-tuba is a vinegar variety derived by fermenting the sap of a coconut tree. You are tasked to analyze its acetic acid
- Describe the preparation of 5.0 M NaOH stock solution from analytical grade NaOH pellets.
- Describe the preparation of 0.10 M NaOH solution from the stock solution.
- How much KHP was used per trial. (purity KHP: 99.0%).
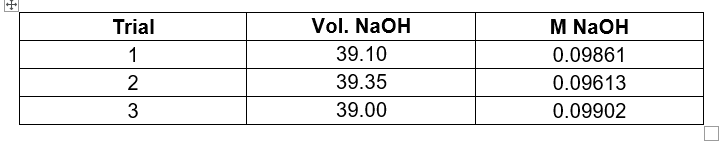
Transcribed Image Text:Trial
Vol. NaOH
M NaOH
1
39.10
0.09861
2
39.35
0.09613
39.00
0.09902
N3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

