Sulfuric acid, H₂SO, con be neutralized by sodium hydroxide, NaOH. The unbalanced equation is: H₂SO4 + NOOH-Na₂SO4+H₂O A student who was asked to balance the reaction wrote the following: H₂SO4) No₂OH(+No₂SO4+H₂O Is this correct? Explain why or why not using what you know about the low of conservation of mass and chemical changes. If necessary provide the correct balanced equation
Sulfuric acid, H₂SO, con be neutralized by sodium hydroxide, NaOH. The unbalanced equation is: H₂SO4 + NOOH-Na₂SO4+H₂O A student who was asked to balance the reaction wrote the following: H₂SO4) No₂OH(+No₂SO4+H₂O Is this correct? Explain why or why not using what you know about the low of conservation of mass and chemical changes. If necessary provide the correct balanced equation
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter6: Chemical Reactions: An Introduction
Section: Chapter Questions
Problem 20QAP: Many over-the-counter antacid tablets are now formulated using calcium carbonate as the active...
Related questions
Question
Subject:
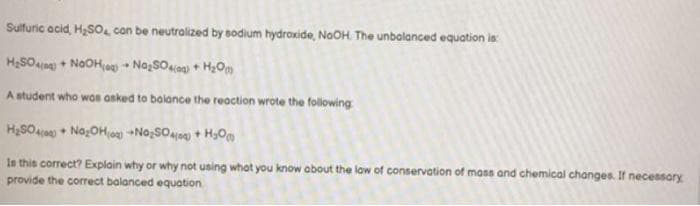
Transcribed Image Text:Sulfuric acid, H₂SO, con be neutralized by sodium hydroxide, NaOH. The unbalanced equation is:
H₂SO4(0) + NOOH(a)Na₂SO4(0) + H₂O
A student who was asked to balance the reaction wrote the following
H₂SO4(0)Na₂OH(0) No₂SO4+H₂O
Is this correct? Explain why or why not using what you know about the law of conservation of mass and chemical changes. If necessary
provide the correct balanced equation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning