Sunflower oil contains 0.090 mol palmitic acid (C₁6H320₂)/mol, 0.060 mol stearic acid (C₁8H360₂)/mol, 0.36 mol oleic acid (C₁8H340₂)/mol, and 0.49 mol linoleic acid (C₁8H320₂)/mol. To create margarine from sunflower oil, the liquid oleic and linoleic acids are hydrogenated in the presence of a metal catalyst to form solid stearic acid. C18H34O2 + H₂C18H3602 C18H3202 + 2H₂ → C18H3602 - At a margarine production facility, a stream of 305.0 mol sunflower oil/h is fed into a reactor. The fresh hydrogen source available is a mixture containing 0.97 mol H₂/mol and 0.030 mol N₂/mol. N₂ is inert in this process. To ensure complete hydrogenation, the hydrogen gas mixture is introduced into the reactor in 55.0% excess. All reaction products leave the reactor and proceed to a separator where palmitic and stearic acids are separated from the hydrogen gas mixture. The hydrogen gas mixture is recycled to the fresh feed stream, and 105.0 mol gas/h of this recycle stream is purged to prevent the buildup of nitrogen gas. What is the molar flow rate of the fresh hydrogen and nitrogen gas mixture to the process?
Sunflower oil contains 0.090 mol palmitic acid (C₁6H320₂)/mol, 0.060 mol stearic acid (C₁8H360₂)/mol, 0.36 mol oleic acid (C₁8H340₂)/mol, and 0.49 mol linoleic acid (C₁8H320₂)/mol. To create margarine from sunflower oil, the liquid oleic and linoleic acids are hydrogenated in the presence of a metal catalyst to form solid stearic acid. C18H34O2 + H₂C18H3602 C18H3202 + 2H₂ → C18H3602 - At a margarine production facility, a stream of 305.0 mol sunflower oil/h is fed into a reactor. The fresh hydrogen source available is a mixture containing 0.97 mol H₂/mol and 0.030 mol N₂/mol. N₂ is inert in this process. To ensure complete hydrogenation, the hydrogen gas mixture is introduced into the reactor in 55.0% excess. All reaction products leave the reactor and proceed to a separator where palmitic and stearic acids are separated from the hydrogen gas mixture. The hydrogen gas mixture is recycled to the fresh feed stream, and 105.0 mol gas/h of this recycle stream is purged to prevent the buildup of nitrogen gas. What is the molar flow rate of the fresh hydrogen and nitrogen gas mixture to the process?
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
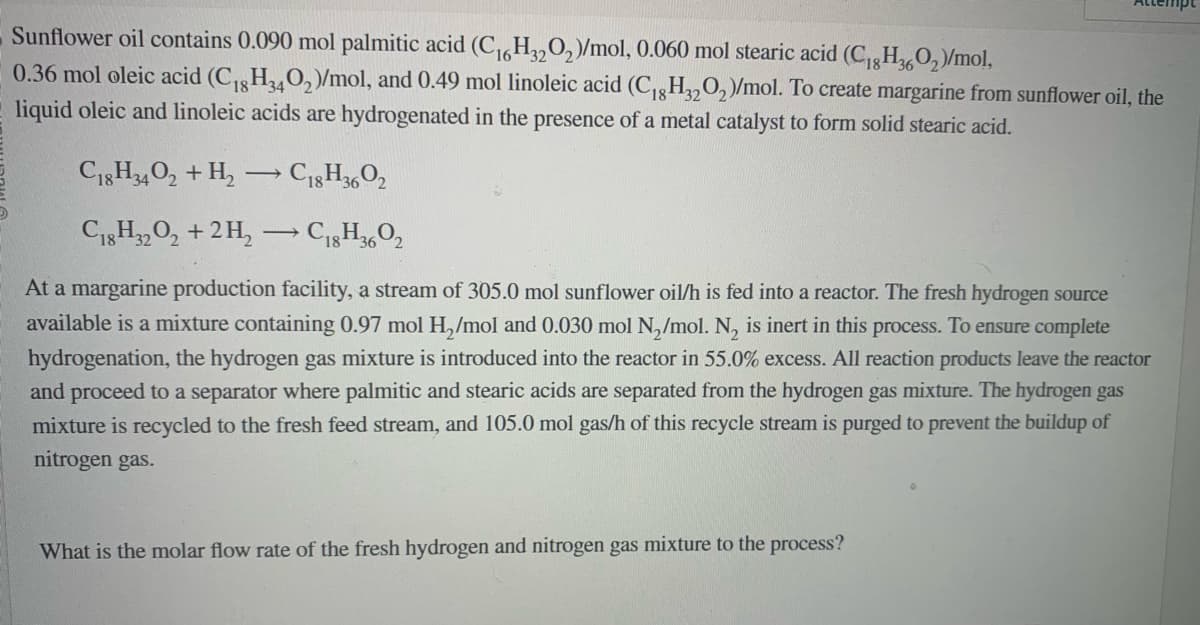
Transcribed Image Text:Sunflower oil contains 0.090 mol palmitic acid (C₁6H3202)/mol, 0.060 mol stearic acid (C₁8H360₂)/mol,
0.36 mol oleic acid (C₁8H340₂)/mol, and 0.49 mol linoleic acid (C₁8H32O₂)/mol. To create margarine from sunflower oil, the
liquid oleic and linoleic acids are hydrogenated in the presence of a metal catalyst to form solid stearic acid.
C18H34O2 + H₂ →→ C18H3602
-
C18H32O2 + 2H₂
→ C18H3602
At a margarine production facility, a stream of 305.0 mol sunflower oil/h is fed into a reactor. The fresh hydrogen source
available is a mixture containing 0.97 mol H₂/mol and 0.030 mol N₂/mol. N₂ is inert in this process. To ensure complete
hydrogenation, the hydrogen gas mixture is introduced into the reactor in 55.0% excess. All reaction products leave the reactor
and proceed to a separator where palmitic and stearic acids are separated from the hydrogen gas mixture. The hydrogen gas
mixture is recycled to the fresh feed stream, and 105.0 mol gas/h of this recycle stream is purged to prevent the buildup of
nitrogen gas.
What is the molar flow rate of the fresh hydrogen and nitrogen gas mixture to the process?
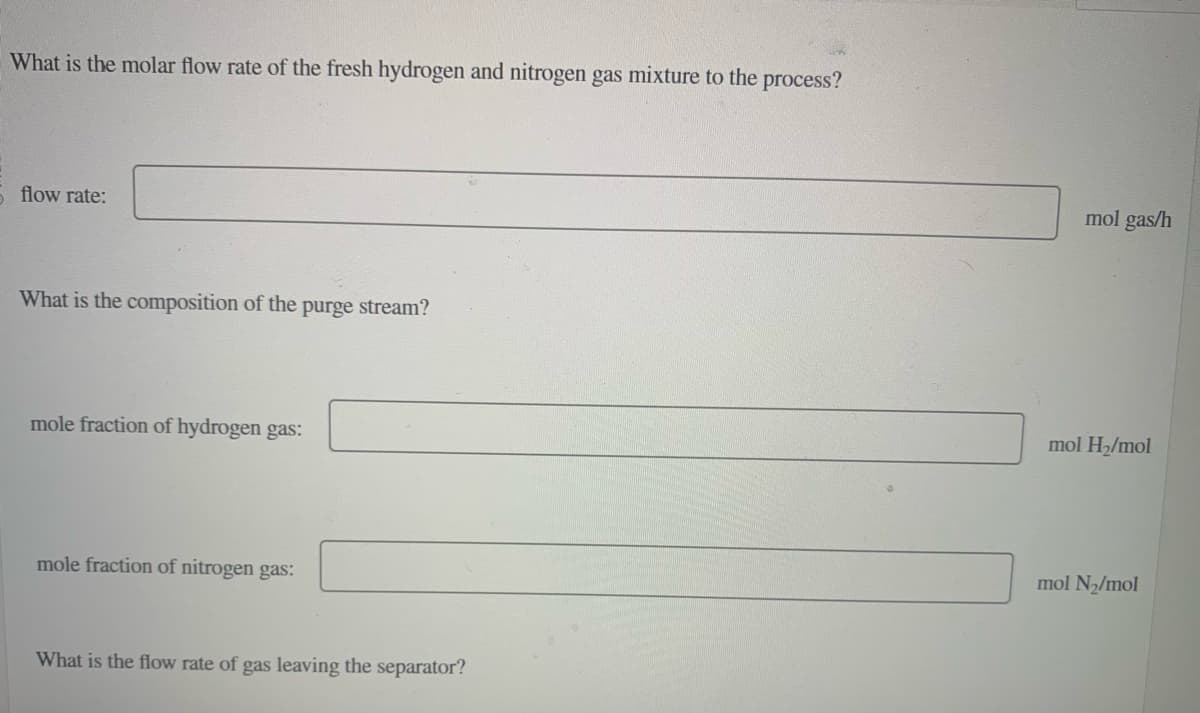
Transcribed Image Text:What is the molar flow rate of the fresh hydrogen and nitrogen gas mixture to the process?
5 flow rate:
What is the composition of the
purge stream?
mole fraction of hydrogen gas:
mole fraction of nitrogen gas:
What is the flow rate of gas leaving the separator?
mol gas/h
mol H₂/mol
mol N₂/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The