Super Ninja has a 10.00 mL sample of 0.15 M of chlorous acid, HClO2(aq). 1)This acid is titrated with 0.20 M sodium hydroxide, NaOH. (i) Calculate the initial pH of the acid before titrating. (ii) Calculate the pH at the equivalence point. (iii) what indicator would you choose for this titration? explain why?
Super Ninja has a 10.00 mL sample of 0.15 M of chlorous acid, HClO2(aq). 1)This acid is titrated with 0.20 M sodium hydroxide, NaOH. (i) Calculate the initial pH of the acid before titrating. (ii) Calculate the pH at the equivalence point. (iii) what indicator would you choose for this titration? explain why?
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter30: Nutrition
Section: Chapter Questions
Problem 30.60P
Related questions
Question
Super Ninja has a 10.00 mL sample of 0.15 M of chlorous acid, HClO2(aq).
1)This acid is titrated with 0.20 M sodium hydroxide, NaOH.
(i) Calculate the initial pH of the acid before titrating.
(ii) Calculate the pH at the equivalence point.
(iii) what indicator would you choose for this titration? explain why?
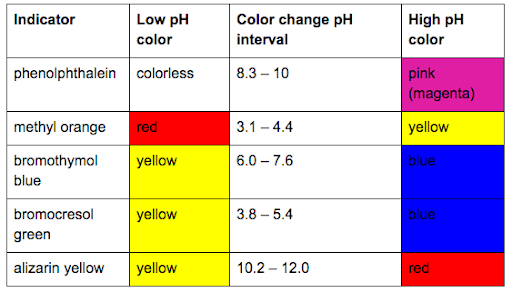
Transcribed Image Text:Indicator
Low pH
Color change pH
High pH
color
interval
color
phenolphthalein colorless
8.3 – 10
pink
(magenta)
methyl orange
red
3.1 – 4.4
yellow
bromothymol
yellow
6.0 – 7.6
blue
blue
bromocresol
yellow
3.8 – 5.4
blue
green
alizarin yellow
yellow
10.2 – 12.0
red
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning