Suppose a 250. mL flask is filled with 1.0 mol of NO, and 1.3 mol of NO. This reaction becomes possible: 2NO, (g) = 2NO (g) + O,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O,. You can leave out the M symbol for molarity. NO, NO 믐 initial change equilibrium
Suppose a 250. mL flask is filled with 1.0 mol of NO, and 1.3 mol of NO. This reaction becomes possible: 2NO, (g) = 2NO (g) + O,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of O,. You can leave out the M symbol for molarity. NO, NO 믐 initial change equilibrium
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter14: Chemical Equilibirum
Section: Chapter Questions
Problem 14.19QP: During an experiment with the Haber process, a researcher put 1 mol N2 and 1 mol H2 into a reaction...
Related questions
Question
100%
kinetics and equilibrium
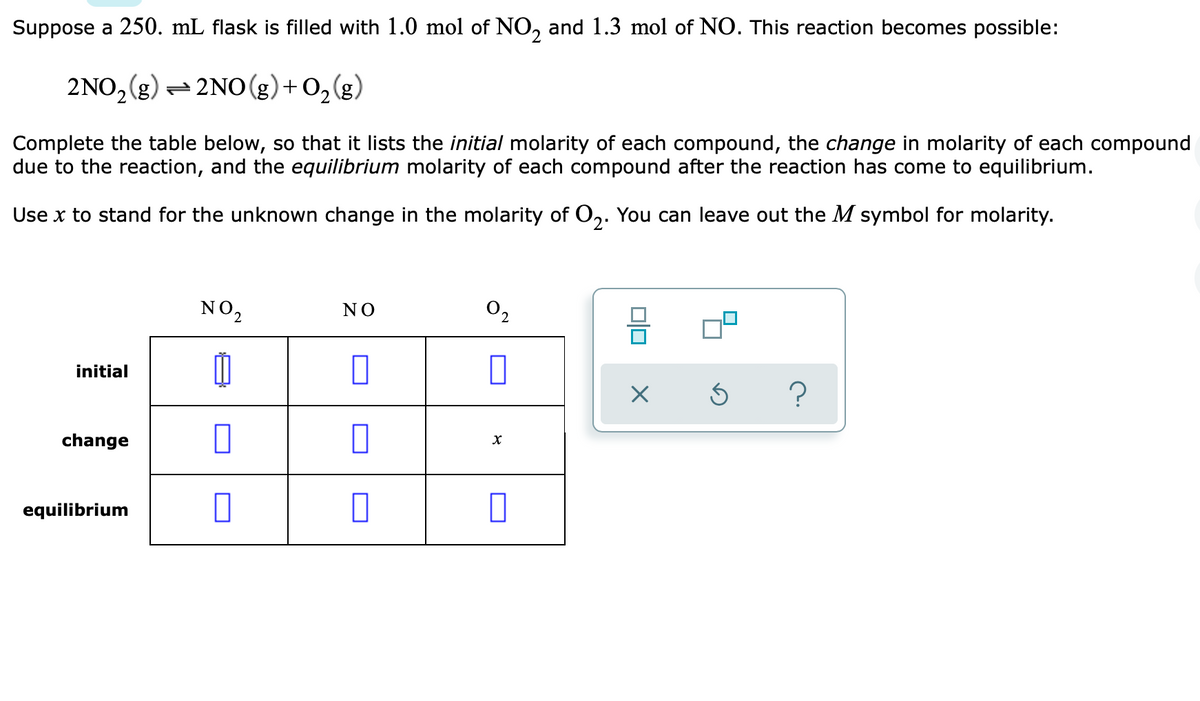
Transcribed Image Text:Suppose a 250. mL flask is filled with 1.0 mol of NO, and 1.3 mol of NO. This reaction becomes possible:
2NO, (g) = 2NO (g)+ O,(g)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound
due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of O,. You can leave out the M symbol for molarity.
NO2
02
NO
initial
change
equilibrium
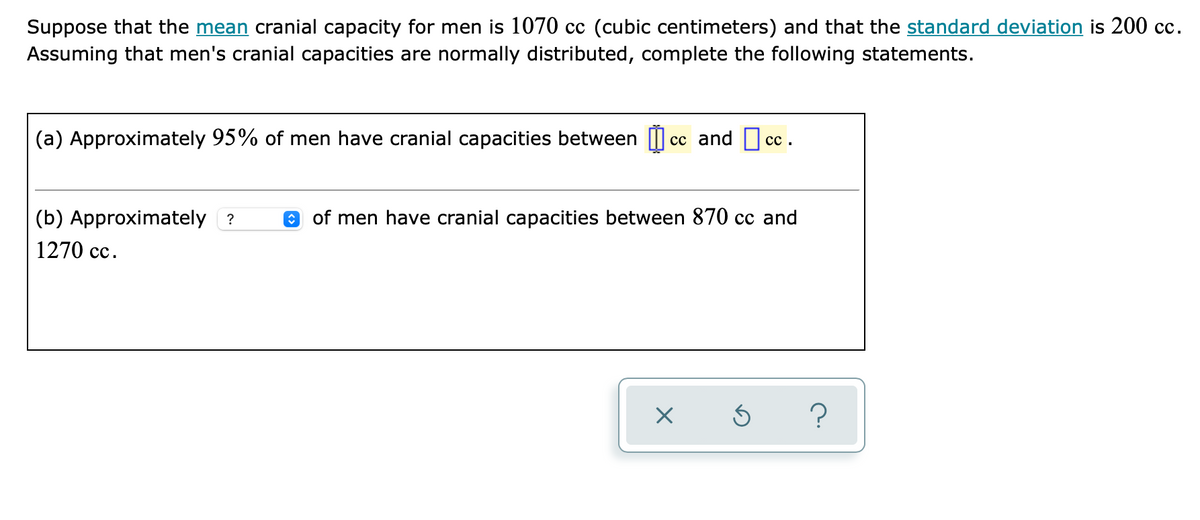
Transcribed Image Text:Suppose that the mean cranial capacity for men is 1070 cc (cubic centimeters) and that the standard deviation is 200 cc.
Assuming that men's cranial capacities are normally distributed, complete the following statements.
(a) Approximately 95% of men have cranial capacities between I cc and cc.
сс.
(b) Approximately ?
O of men have cranial capacities between 870 cc and
1270 cc.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning