While ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 50 L tank with 9.0 mol of ethylene gas and 11. mol of water vapor. When the mixture has come to equilibrium he determines that it contains 4.9 mol of ethylene gas and 6.9 mol of water vapor. The engineer then adds another 3.0 mol of ethylene, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. mol
While ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 50 L tank with 9.0 mol of ethylene gas and 11. mol of water vapor. When the mixture has come to equilibrium he determines that it contains 4.9 mol of ethylene gas and 6.9 mol of water vapor. The engineer then adds another 3.0 mol of ethylene, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. mol
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 54P
Related questions
Question
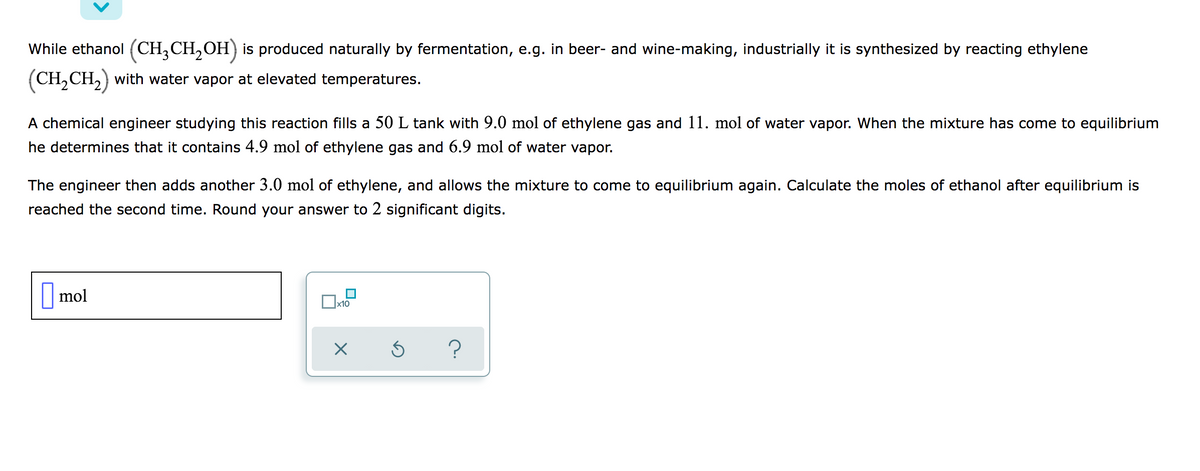
Transcribed Image Text:While ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene
(CH,CH,) with water vapor at elevated temperatures.
A chemical engineer studying this reaction fills a 50 L tank with 9.0 mol of ethylene gas and 11. mol of water vapor. When the mixture has come to equilibrium
he determines that it contains 4.9 mol of ethylene gas and 6.9 mol of water vapor.
The engineer then adds another 3.0 mol of ethylene, and allows the mixture to come to equilibrium again. Calculate the moles of ethanol after equilibrium is
reached the second time. Round your answer to 2 significant digits.
|mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning