Suppose a 250. mL flask is filled with 1.8 mol of H,O, 0.30 mol of CO and 0.10 mol of H,. This reaction becomes possible: CH,(2) + H,O(2) - CO(2)+3H,(3) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of H,0. You can leave out the M symbol for molarity. CH, H.O CO H. initial change equilibrium
Suppose a 250. mL flask is filled with 1.8 mol of H,O, 0.30 mol of CO and 0.10 mol of H,. This reaction becomes possible: CH,(2) + H,O(2) - CO(2)+3H,(3) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of H,0. You can leave out the M symbol for molarity. CH, H.O CO H. initial change equilibrium
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 20Q: A student wants to prepare 1.00 L of a 1.00-M solution of NaOH (molar mass = 40.00 g/mol). If solid...
Related questions
Question
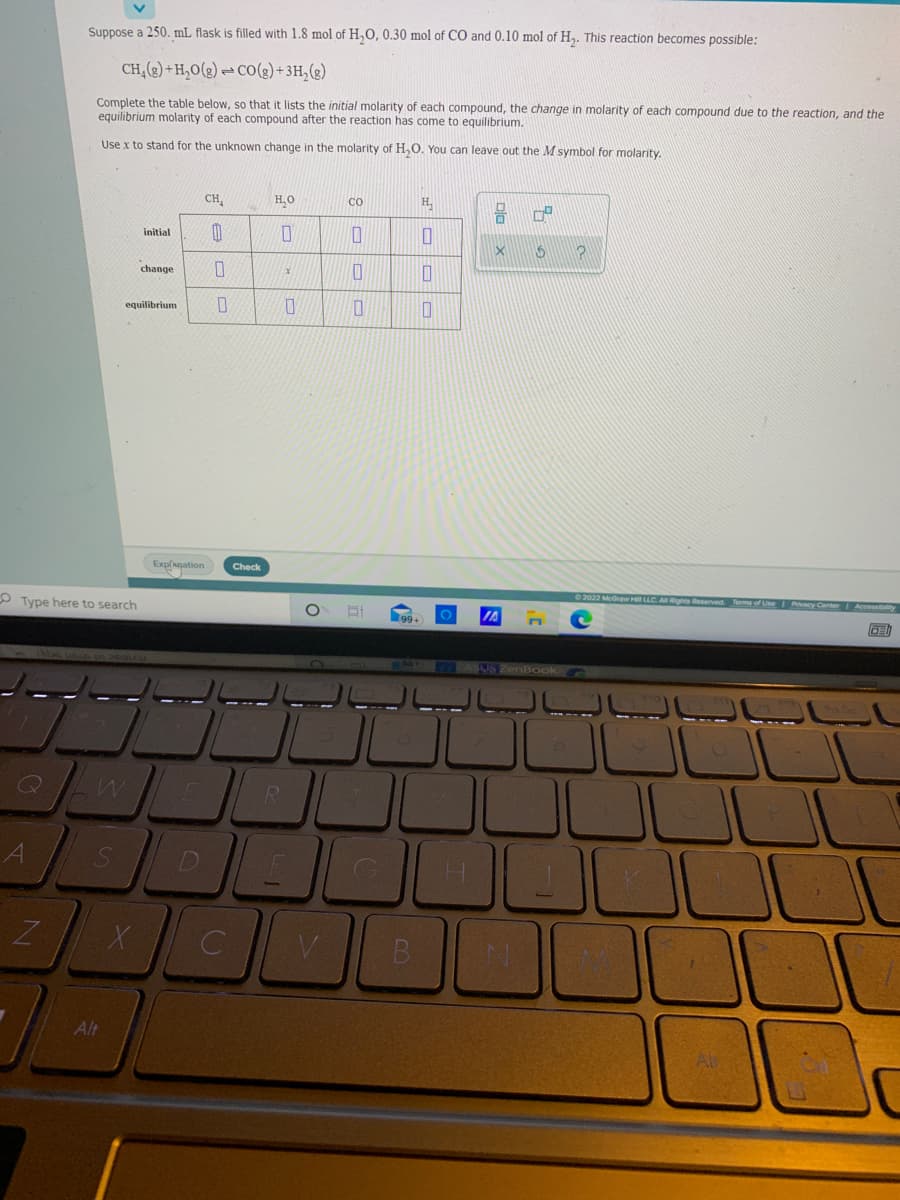
Transcribed Image Text:Suppose a 250. mL flask is filled with 1.8 mol of H,0, 0.30 mol of CO and 0.10 mol of H,. This reaction becomes possible:
CH,(2) + H,O(g) → CO(g) + 3H,(g)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the
equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of H,O. You can leave out the M symbol for molarity.
CH,
H,O
H,
CO
initial
change
equilibrium
Explagation
Check
O Type here to search
2022 McGraw Hl LLC A Rights Reserved Terms of Use Pvacy Center Accessibaty
US ZenBook
Q
R
Alt
Dla X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning