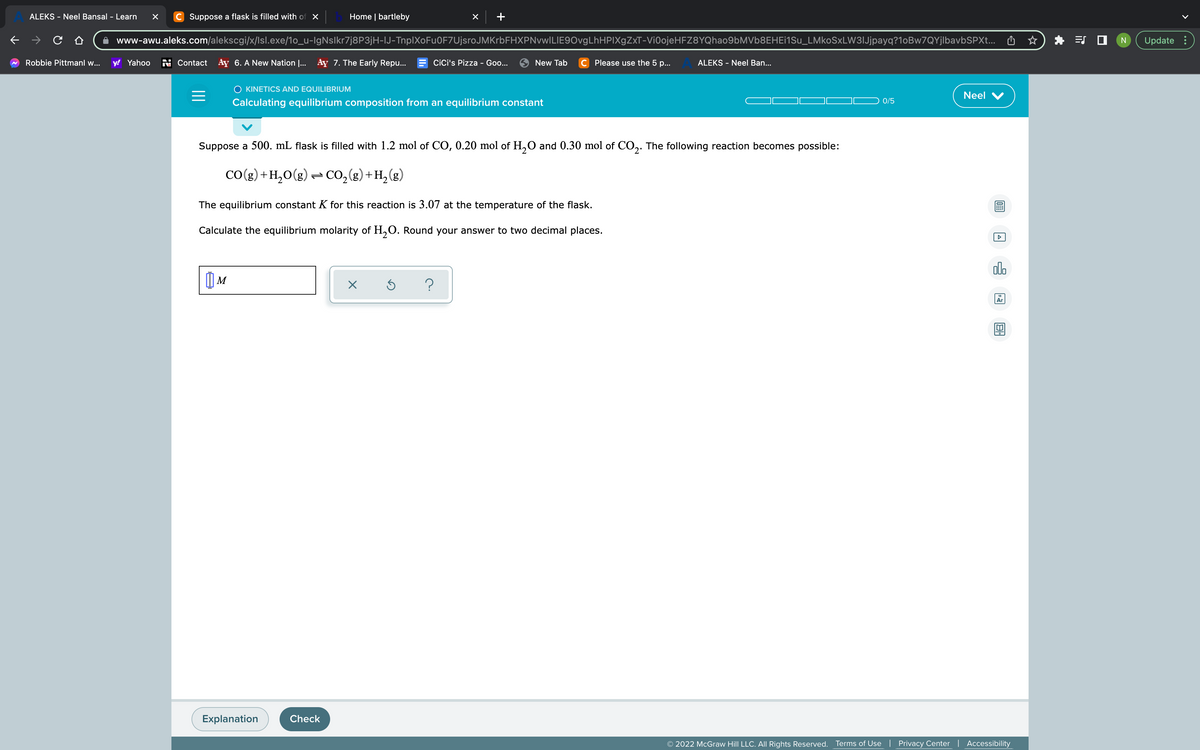
Suppose a 500. mL flask is filled with 1.2 mol of CO, 0.20 mol of H₂O and 0.30 mol of CO₂. The following reaction becomes possible: CO(g) + H₂O(g) + CO₂(g) + H₂(g) The equilibrium constant K for this reaction is 3.07 at the temperature of the flask. Calculate the equilibrium molarity of H₂O. Round your answer to two decimal places. M X
Suppose a 500. mL flask is filled with 1.2 mol of CO, 0.20 mol of H₂O and 0.30 mol of CO₂. The following reaction becomes possible: CO(g) + H₂O(g) + CO₂(g) + H₂(g) The equilibrium constant K for this reaction is 3.07 at the temperature of the flask. Calculate the equilibrium molarity of H₂O. Round your answer to two decimal places. M X
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
I know how to solve it but want to make sure i did it right thx
Transcribed Image Text:←
ALEKS - Neel Bansal - Learn X C Suppose a flask is filled with of X
C D
Robbie Pittmanl w... y Yahoo
www-awu.aleks.com/alekscgi/x/lIsl.exe/1o_u-lgNslkr7j8P3jH-IJ-TnplXoFu0F7UjsroJMKrbFHXPNvwILIE9OvgLhHPIXgZxT-Vi0ojeHFZ8YQhao9bMVb8EHEi1Su_LMkoSxLW3IJjpayq?10Bw7QYjlbavbSPXt...
New Tab C Please use the 5 p...
Contact Ay 6. A New Nation ... Ay 7. The Early Repu...
=
Home | bartleby
M
O KINETICS AND EQUILIBRIUM
Calculating equilibrium composition from an equilibrium constant
Explanation
×
Suppose a 500. mL flask is filled with 1.2 mol of CO, 0.20 mol of H₂O and 0.30 mol of CO2. The following reaction becomes possible:
CO(g) + H₂O(g) → CO₂(g) + H₂(g)
The equilibrium constant K for this reaction is 3.07 at the temperature of the flask.
Calculate the equilibrium molarity of H₂O. Round your answer to two decimal places.
Check
CiCi's Pizza - Goo...
x 5
?
ALEKS - Neel Ban...
0/5
Neel V
0:
olo
Ar
Al
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
* = N
Update :
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,



Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
