Suppose a 500. mL flask is filled with 1.8 mol of CH,, 0.60 mol of H,S and 0.10 mol of H,. This reaction becomes possible: CH,(2) + 2H,S(g) → Cs,(e) +4H,(2) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reacti equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CS,. You can leave out the M symbol for molarity. CH, H,S cs, H, initial 3.6 1.2 5 ? change equilibrium
Suppose a 500. mL flask is filled with 1.8 mol of CH,, 0.60 mol of H,S and 0.10 mol of H,. This reaction becomes possible: CH,(2) + 2H,S(g) → Cs,(e) +4H,(2) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reacti equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CS,. You can leave out the M symbol for molarity. CH, H,S cs, H, initial 3.6 1.2 5 ? change equilibrium
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 17QAP: What is the molarity of each ion present in aqueous solutions of the following compounds prepared by...
Related questions
Question
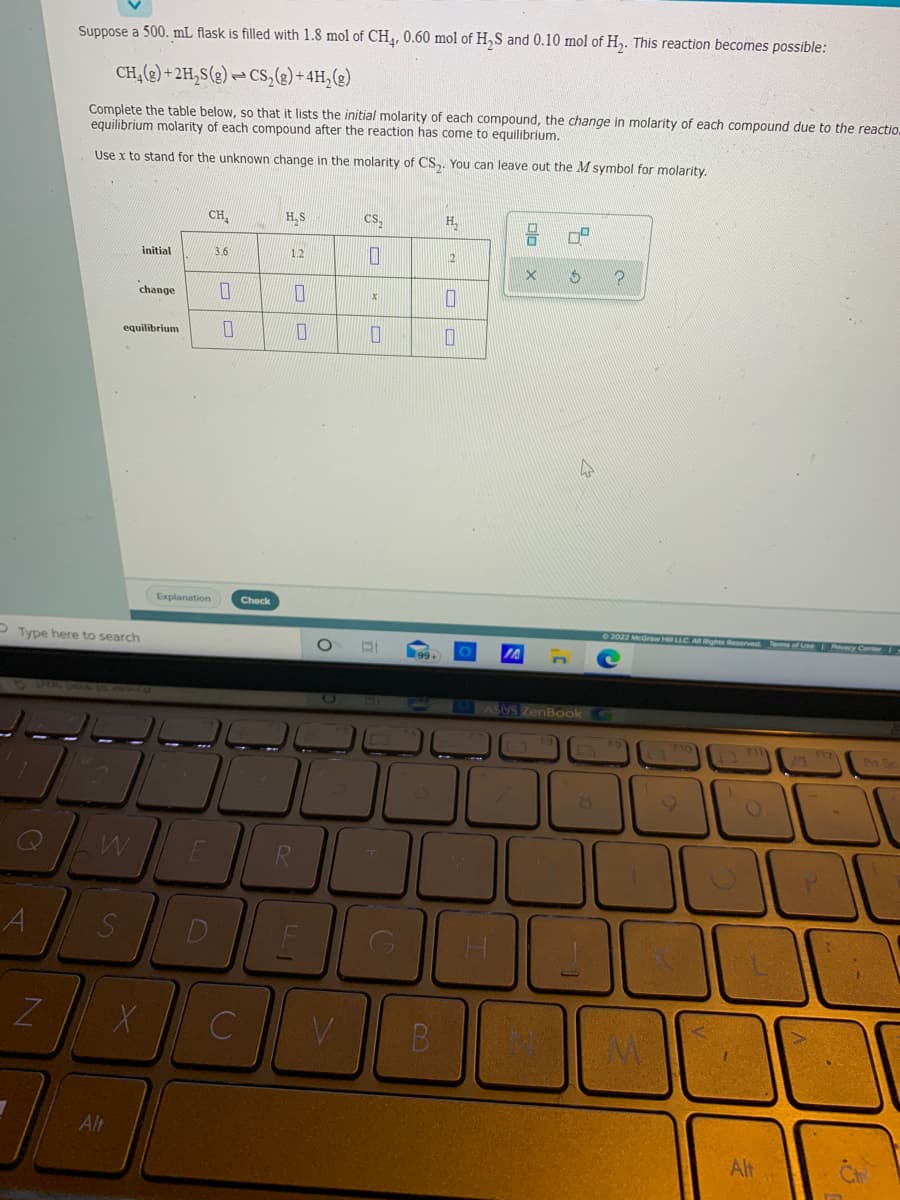
Transcribed Image Text:Suppose a 500. mL flask is filled with 1.8 mol of CH,, 0.60 mol of H,S and 0.10 mol of H,. This reaction becomes possible:
CH,(2) + 2H,S(g) → Cs,(g) +4H,(2)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reactio.
equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of CS,. You can leave out the M symbol for molarity.
CH,
H,S
Cs,
H,
initial
3.6
12
2
change
equilibrium
Explanation
Check
2022 McGraw H LLC A ights eser
O Type here to search
SPOG URAL
SUs ZenBook
D
Alt
Alt
Expert Solution
Step 1
The equilibrium reaction given is,
Given: Volume of flask = 0.500 L (Since 1 L = 1000 mL)
Moles of CH4 initially = 1.8 mol.
Moles of H2S initially = 0.60 mol.
Moles of H2 initially = 0.10 mol.
And the change in molarity of CS2 = x.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
