Suppose a limekiln of volume 750. L is pressurized with carbon dioxide gas to 10.8 atm, and heated to 1140. °C. When the amount of CO, has stopped 2 changing, it is found that 2.59 kg of CaCO, have appeared. Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaC0, and CaO at 1140. °C. Round your answer to 2 significant digits.
Suppose a limekiln of volume 750. L is pressurized with carbon dioxide gas to 10.8 atm, and heated to 1140. °C. When the amount of CO, has stopped 2 changing, it is found that 2.59 kg of CaCO, have appeared. Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaC0, and CaO at 1140. °C. Round your answer to 2 significant digits.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter5: Gases
Section: Chapter Questions
Problem 162CP
Related questions
Question
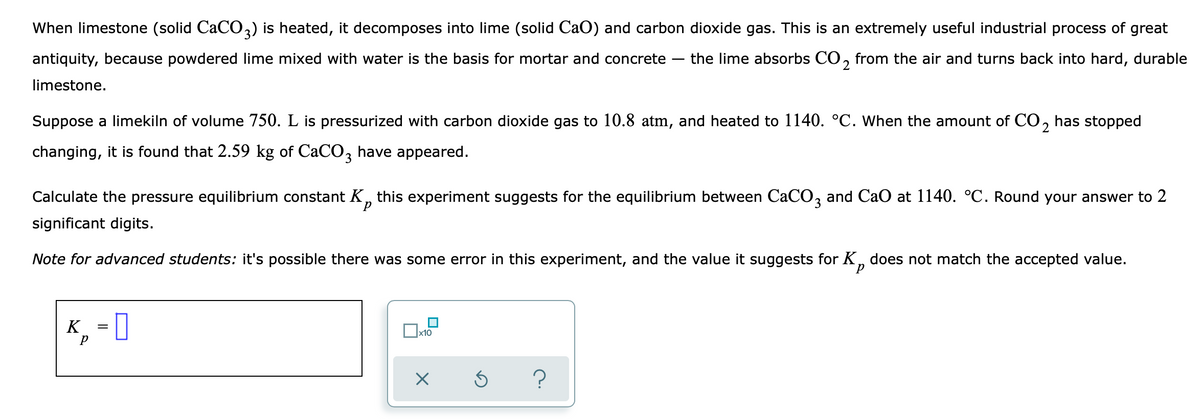
Transcribed Image Text:When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great
antiquity, because powdered lime mixed with water is the basis for mortar and concrete-
the lime absorbs CO, from the air and turns back into hard, durable
limestone.
Suppose a limekiln of volume 750. L is pressurized with carbon dioxide gas to 10.8 atm, and heated to 1140. °C. When the amount of CO, has stopped
changing, it is found that 2.59 kg of CaCO, have appeared.
Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaCO, and Cao at 1140. °C. Round your answer to 2
d.
significant digits.
Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K, does not match the accepted value.
K, = [
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning