At 500 K, we have the data Substance AH300/(kJ/mol) S3o0/(J/K mol) HI(g) H2(g) I,(g) 32.41 221.63 5.88 145.64 69.75 279.94 One mole of H,.and one mole of I2 are placed in a vessel at 500 K. At this temperature only gases are present and the equilibrium H2(g) + I2(g) 2 HI(g) is established. Calculate K, at 500 K and the mole fraction of HI present at 500 K and 1 atm. What would the mole fraction of HI be at 500 K and 10 atm?
At 500 K, we have the data Substance AH300/(kJ/mol) S3o0/(J/K mol) HI(g) H2(g) I,(g) 32.41 221.63 5.88 145.64 69.75 279.94 One mole of H,.and one mole of I2 are placed in a vessel at 500 K. At this temperature only gases are present and the equilibrium H2(g) + I2(g) 2 HI(g) is established. Calculate K, at 500 K and the mole fraction of HI present at 500 K and 1 atm. What would the mole fraction of HI be at 500 K and 10 atm?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.35E: A piston having 7.23 g of steam at 110 C increases its temperature by 35 C. At the same time, it...
Related questions
Question
The answer for Kp= 12.9 and the answer for x=0.842. Please show me how to obtain this answers
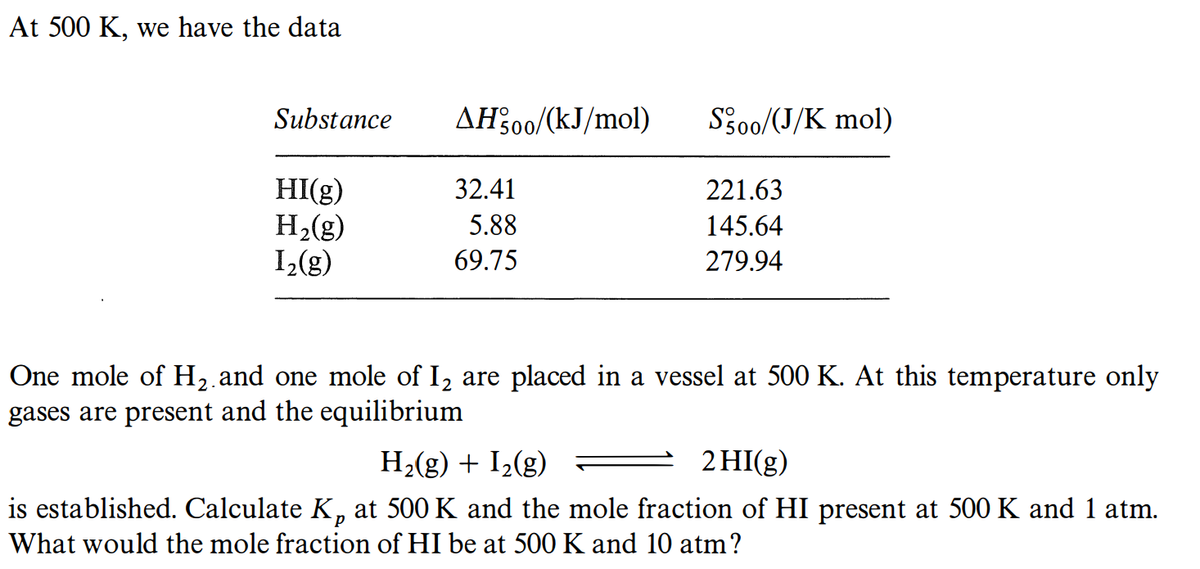
Transcribed Image Text:At 500 K, we have the data
Substance
AH200/(kJ/mol)
S3o0/(J/K mol)
HI(g)
H2(g)
1(g)
32.41
221.63
5.88
145.64
69.75
279.94
One mole of H2.and one mole of I2 are placed in a vessel at 500 K. At this temperature only
gases are present and the equilibrium
H2(g) + I2(g)
2 HI(g)
is established. Calculate K, at 500 K and the mole fraction of HI present at 500 K and 1 atm.
What would the mole fraction of HI be at 500 K and 10 atm?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning