Suppose a reaction A+B C occurs at some initial rate at 25°C. Which response includes all of the changes below that could increase the rate of this reaction? I. II. lowering the temperature increasing the initial concentration of B adding a catalyst III. (a) I (b) II (с) I (d) I and II (e) II and III
Suppose a reaction A+B C occurs at some initial rate at 25°C. Which response includes all of the changes below that could increase the rate of this reaction? I. II. lowering the temperature increasing the initial concentration of B adding a catalyst III. (a) I (b) II (с) I (d) I and II (e) II and III
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.29E
Related questions
Question
kindly answer the following question below, choose the best answer. thank u so much

Transcribed Image Text:61.
Which is not an example of the effect of subdivision of the reactant on the rate of
chemical reaction?
(a)
Violent explosions that occur in grain elevators.
A container of flammable liquid will burn on the surface but allowed to vaporize
will burn explosively.
A chunk of iron takes months to rust completely while iron wool will rust in days.
Some metals may be fused (welded) with minimal loss while their powders will
burn in a flame.
(b)
(c)
(d)
(e)
The Grand Canyon was created by dissolution by water over millions of years.
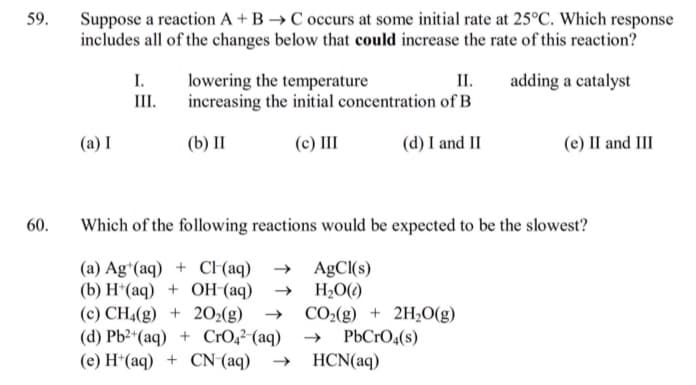
Transcribed Image Text:59.
Suppose a reaction A + B →C occurs at some initial rate at 25°C. Which response
includes all of the changes below that could increase the rate of this reaction?
I.
III.
II.
lowering the temperature
increasing the initial concentration of B
adding a catalyst
(a) I
(b) II
(c) II
(d) I and II
(e) II and III
60.
Which of the following reactions would be expected to be the slowest?
(a) Ag*(aq) + C (aq) → AgCI(s)
(b) H*(aq) + OH (aq)
(c) CH4(g) + 202(g)
(d) Pb2*(aq) + CrO,²-(aq)
(e) H*(aq) + CN (aq)
H2O()
CO2(g) + 2H,O(g)
PbCrO4(s)
HCN(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning