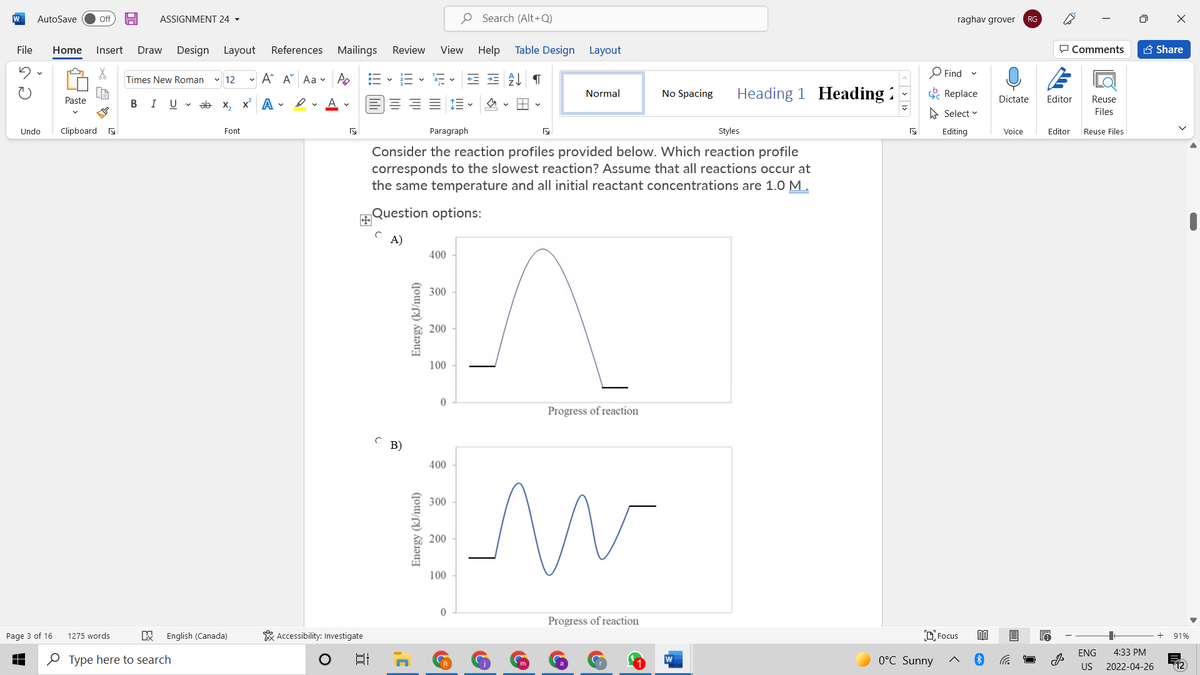
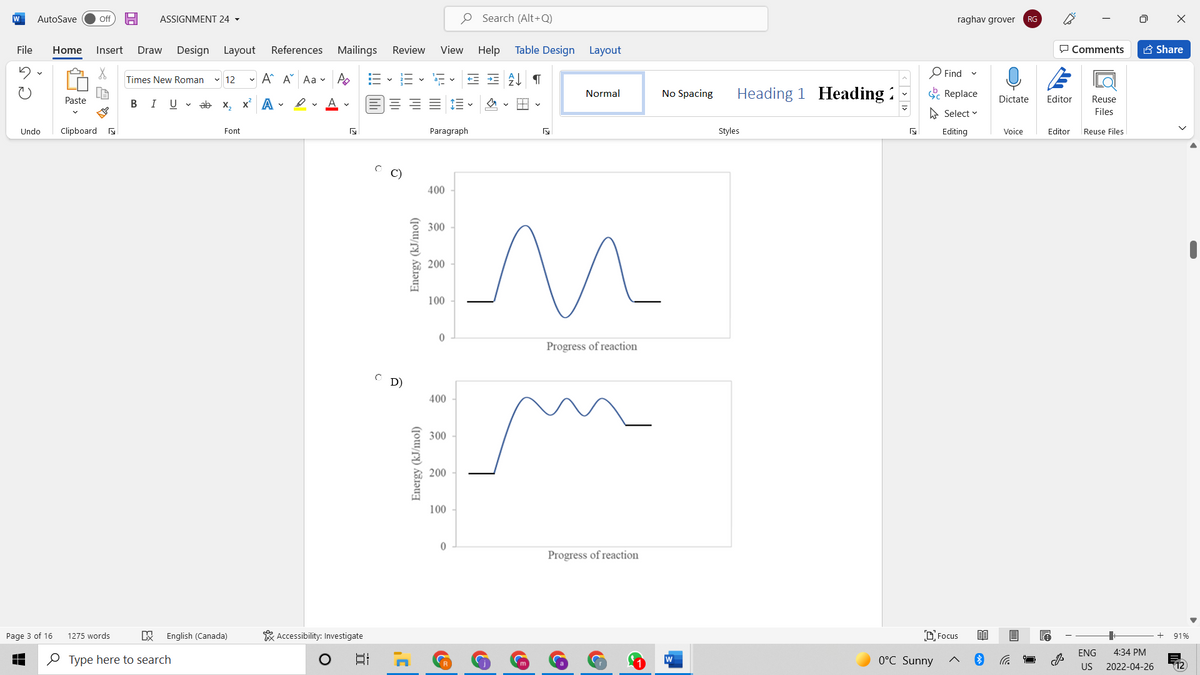
Consider the reaction profiles provided below. Which reaction profile corresponds to the slowest reaction? Assume that all reactions occur at the same temperature and all initial reactant concentrations are 1.0 M. Question options: A) 400 300 200 100 Progress of reaction C B) 400 300 200 100 Energy (kJ/mol) Energy (kJ/mol)
Consider the reaction profiles provided below. Which reaction profile corresponds to the slowest reaction? Assume that all reactions occur at the same temperature and all initial reactant concentrations are 1.0 M. Question options: A) 400 300 200 100 Progress of reaction C B) 400 300 200 100 Energy (kJ/mol) Energy (kJ/mol)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter1: The Atom In Modern Chemistry
Section: Chapter Questions
Problem 11P
Related questions
Question
Transcribed Image Text:W
AutoSave
O Search (Alt+Q)
Off
ASSIGNMENT 24 -
raghav grover
RG
File
Home
Insert
Design Layout
References Mailings
Review
View Help
Table Design Layout
P Comments
A Share
Draw
- A A Aa v Po
O Find
Times New Roman
v 12
Heading 1 Heading :
E Replace
Normal
No Spacing
Paste
В I
U v ab x, x A -
Dictate
Editor
Reuse
A Select v
Files
Undo
Clipboard a
Font
Paragraph
Styles
Editing
Voice
Editor
Reuse Files
Consider the reaction profiles provided below. Which reaction profile
corresponds to the slowest reaction? Assume that all reactions occur at
the same temperature and all initial reactant concentrations are 1.0 M.
Question options:
A)
400
300
200
100
Progress of reaction
C B)
400
300
200
100
Progress of reaction
Page 3 of 16
1275 words
English (Canada)
* Accessibility: Investigate
D Focus
91%
ENG
4:33 PM
O Type here to search
日
0°C Sunny
W
US
2022-04-26
12
Energy (kJ/mol)
Transcribed Image Text:W
AutoSave
O Search (Alt+Q)
Off
ASSIGNMENT 24 -
raghav grover
RG
File
Home
Insert
Draw
Design Layout
References Mailings
Review
View Help
Table Design Layout
P Comments
A Share
- A A Aa v Po
O Find
Times New Roman
v 12
No Spacing
Heading 1 Heading :
E Replace
Normal
Paste
В I
U v ab x, x A -
Dictate
Editor
Reuse
A Select v
Files
Undo
Clipboard a
Font
Paragraph
Styles
Editing
Voice
Editor
Reuse Files
C)
400
EM
300
200
100
Progress of reaction
D)
400
300
200
100
Progress of reaction
Page 3 of 16
1275 words
English (Canada)
* Accessibility: Investigate
D Focus
91%
ENG
4:34 PM
O Type here to search
日
0°C Sunny
W
US
2022-04-26
12
Energy (kJ/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning