Suppose H2(g) and I(g) are sealed in a flask at T = 400 K with partial pressures PH, = 1.320 atm and R, = 1.140 atm. At this temperature H, and I, do not react rap- idly to form HI(g), although after a long enough time they would produce HI(g) at its equilibrium partial pressure. Suppose, instead, that the gases are heated in the sealed flask to 600 K, a temperature at which they quickly reach equilibrium: H2 (g) + I2(g)=2 HI(g) The equilibrium constant for the reaction is 92.6 at 600 K: P PH, P, = 92.6 (a) What are the equilibrium values of R1,, P., and PHI at 600 K? (b) What percentage of the I, originally present has reacted when equilibrium is reached?
Suppose H2(g) and I(g) are sealed in a flask at T = 400 K with partial pressures PH, = 1.320 atm and R, = 1.140 atm. At this temperature H, and I, do not react rap- idly to form HI(g), although after a long enough time they would produce HI(g) at its equilibrium partial pressure. Suppose, instead, that the gases are heated in the sealed flask to 600 K, a temperature at which they quickly reach equilibrium: H2 (g) + I2(g)=2 HI(g) The equilibrium constant for the reaction is 92.6 at 600 K: P PH, P, = 92.6 (a) What are the equilibrium values of R1,, P., and PHI at 600 K? (b) What percentage of the I, originally present has reacted when equilibrium is reached?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 82AP
Related questions
Question
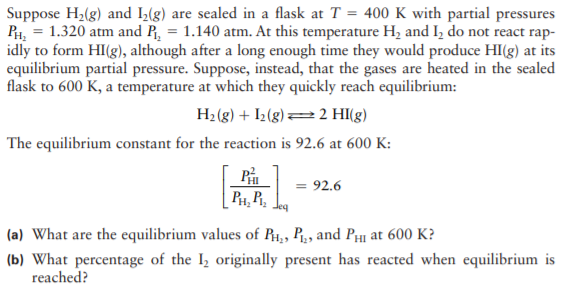
Transcribed Image Text:Suppose H2(g) and I(g) are sealed in a flask at T = 400 K with partial pressures
PH, = 1.320 atm and R, = 1.140 atm. At this temperature H, and I, do not react rap-
idly to form HI(g), although after a long enough time they would produce HI(g) at its
equilibrium partial pressure. Suppose, instead, that the gases are heated in the sealed
flask to 600 K, a temperature at which they quickly reach equilibrium:
H2 (g) + I2(g)=2 HI(g)
The equilibrium constant for the reaction is 92.6 at 600 K:
P
PH, P,
= 92.6
(a) What are the equilibrium values of R1,, P., and PHI at 600 K?
(b) What percentage of the I, originally present has reacted when equilibrium is
reached?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning