Suppose that any given kind of bond, such as 0-H, has a characteristic electric dipole. That is, suppose that elec- tric dipole moments can be assigned to bonds just as bond energies can be. Both are usefully accurate approxi- mations. Consider the water molecule H `H Show that if MOH is the dipole moment of the OH bond, then the dipole moment of water is µ(H2O) = 2µ0H cos (0/2). What is the dipole moment µoH if µ(H,O) is 1.86 D?
Suppose that any given kind of bond, such as 0-H, has a characteristic electric dipole. That is, suppose that elec- tric dipole moments can be assigned to bonds just as bond energies can be. Both are usefully accurate approxi- mations. Consider the water molecule H `H Show that if MOH is the dipole moment of the OH bond, then the dipole moment of water is µ(H2O) = 2µ0H cos (0/2). What is the dipole moment µoH if µ(H,O) is 1.86 D?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 105AP
Related questions
Question
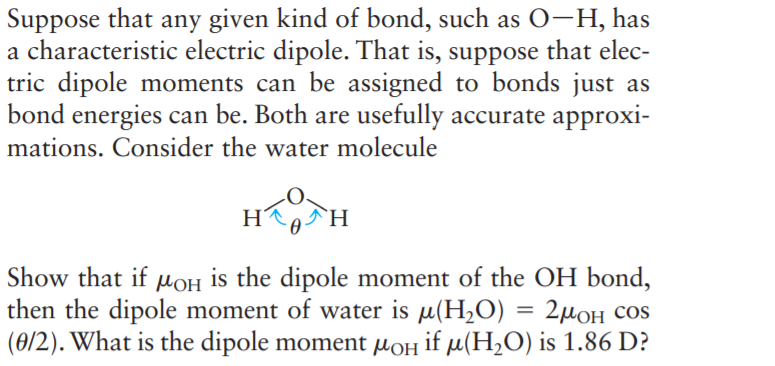
Transcribed Image Text:Suppose that any given kind of bond, such as 0-H, has
a characteristic electric dipole. That is, suppose that elec-
tric dipole moments can be assigned to bonds just as
bond energies can be. Both are usefully accurate approxi-
mations. Consider the water molecule
H
`H
Show that if MOH is the dipole moment of the OH bond,
then the dipole moment of water is µ(H2O) = 2µ0H cos
(0/2). What is the dipole moment µoH if µ(H,O) is 1.86 D?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning