Suppose there are two known compounds containing the generic elements X and Y. You have a 1.00 g sample of each compound. One sample contains 0.28 g of X and the other contains 0.37 g of X.
Suppose there are two known compounds containing the generic elements X and Y. You have a 1.00 g sample of each compound. One sample contains 0.28 g of X and the other contains 0.37 g of X.
Chapter4: Forces Between Particles
Section: Chapter Questions
Problem 4.111E
Related questions
Question
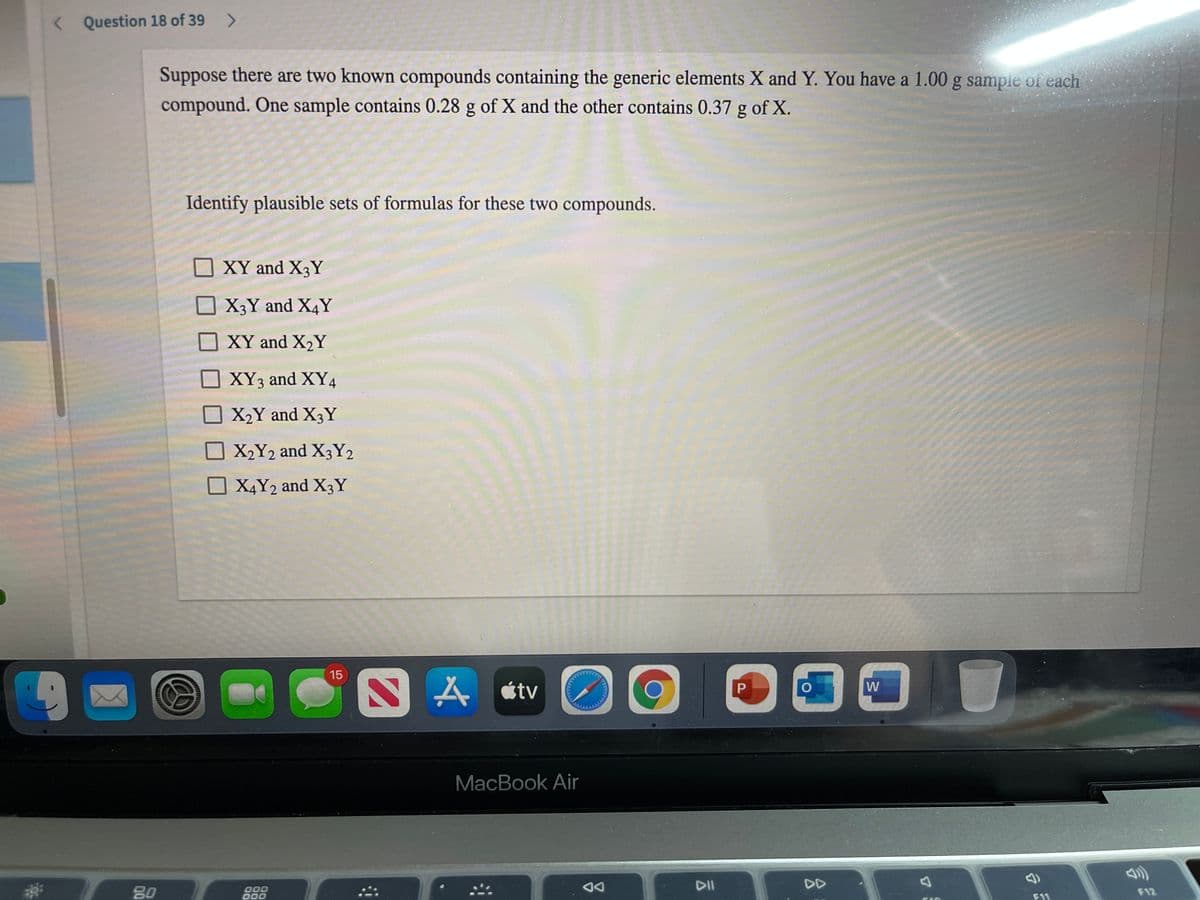
Transcribed Image Text:< Question 18 of 39
Suppose there are two known compounds containing the generic elements X and Y. You have a 1.00 g sample of each
compound. One sample contains 0.28 g of X and the other contains 0.37 g of X.
Identify plausible sets of formulas for these two compounds.
XY and X3Y
X3Y and X4Y
XY and X2Y
XY3 and XY4
X2Y and X3Y
X2Y2 and X3Y2
O X4Y2 and X3Y
15
SA étv
W
MacBook Air
DA
DII
DD
80
000
000
F12
F11
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning