2) a) Write formulas for the figures. Dark circles represent element A and light circles represent element B b) Classify each of the pictures as atomic element, molecular element, compound, mixture of molecular elements, mixture of compounds, mixture of atomic element and compound etc. A figure can have more than one label if needed. Explain your reasoning. a) 8 b) c) d) 8 8
2) a) Write formulas for the figures. Dark circles represent element A and light circles represent element B b) Classify each of the pictures as atomic element, molecular element, compound, mixture of molecular elements, mixture of compounds, mixture of atomic element and compound etc. A figure can have more than one label if needed. Explain your reasoning. a) 8 b) c) d) 8 8
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter4: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 20ALQ: Both atomic elements and molecular elements exist. Are there such entities as atomic compounds and...
Related questions
Concept explainers
Question
Please help me complete this
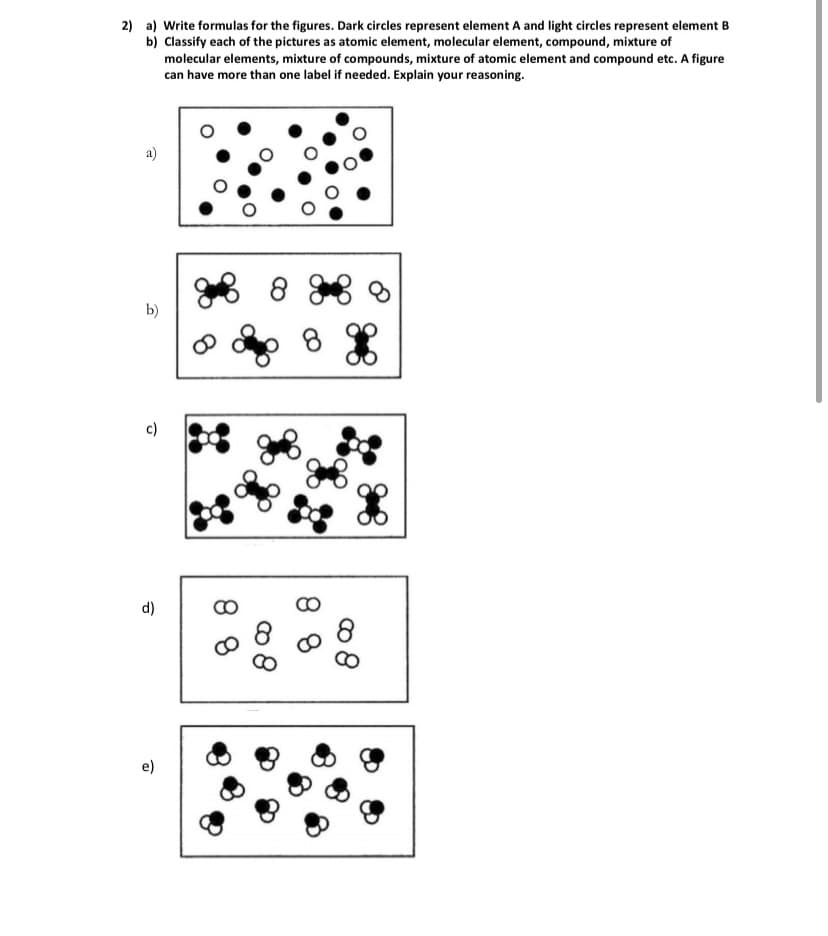
Transcribed Image Text:2) a) Write formulas for the figures. Dark circles represent element A and light circles represent element B
b) Classify each of the pictures as atomic element, molecular element, compound, mixture of
molecular elements, mixture of compounds, mixture of atomic element and compound etc. A figure
can have more than one label if needed. Explain your reasoning.
a)
b)
8.
c)
d)
CO
e)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning