TABLE 18.1 The Major Components of Dry Air near Sea Level Component* Content (mole fraction) Molar Mass (g/mol) Nitrogen 0.78084 28.013 Oxygen 0.20948 31.998 Argon 0.00934 39.948 Carbon dioxide 0.000400 44.0099 Neon 0.00001818 20.183 Helium 0.00000524 4.003 Methane 0.000002 16.043 Krypton 0.00000114 83.80 Hydrogen 0.0000005 2.0159 Nitrous oxide 0.0000005 44.0128 Xenon 0.000000087 131.30 *Ozone, sulfur dioxide, nitrogen dioxide, ammonia, and carbon monoxide are present as trace gases in variable amounts.
TABLE 18.1 The Major Components of Dry Air near Sea Level Component* Content (mole fraction) Molar Mass (g/mol) Nitrogen 0.78084 28.013 Oxygen 0.20948 31.998 Argon 0.00934 39.948 Carbon dioxide 0.000400 44.0099 Neon 0.00001818 20.183 Helium 0.00000524 4.003 Methane 0.000002 16.043 Krypton 0.00000114 83.80 Hydrogen 0.0000005 2.0159 Nitrous oxide 0.0000005 44.0128 Xenon 0.000000087 131.30 *Ozone, sulfur dioxide, nitrogen dioxide, ammonia, and carbon monoxide are present as trace gases in variable amounts.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.109PAE
Related questions
Question
(a) From the data in Table , what is the concentration
of neon in the atmosphere in ppm? (b) What is the concentration
of neon in the atmosphere in molecules per
liter, assuming an atmospheric pressure of 730 torr and a
temperature of 296 K?
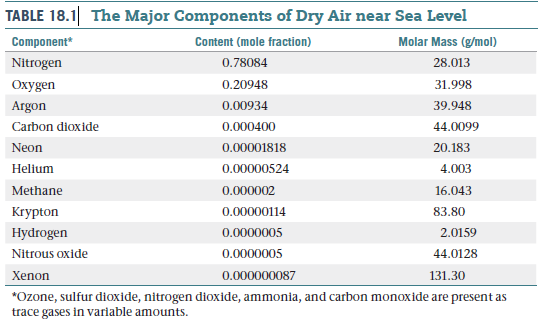
Transcribed Image Text:TABLE 18.1 The Major Components of Dry Air near Sea Level
Component*
Content (mole fraction)
Molar Mass (g/mol)
Nitrogen
0.78084
28.013
Oxygen
0.20948
31.998
Argon
0.00934
39.948
Carbon dioxide
0.000400
44.0099
Neon
0.00001818
20.183
Helium
0.00000524
4.003
Methane
0.000002
16.043
Krypton
0.00000114
83.80
Hydrogen
0.0000005
2.0159
Nitrous oxide
0.0000005
44.0128
Xenon
0.000000087
131.30
*Ozone, sulfur dioxide, nitrogen dioxide, ammonia, and carbon monoxide are present as
trace gases in variable amounts.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning